Receptor antagonist long-term effectiveness analysis method
A receptor antagonist and analysis method technology, applied in the field of long-term analysis of receptor antagonists, can solve the problems of low screening throughput and long test period, achieve high screening throughput, reliable detection results, and improve test efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
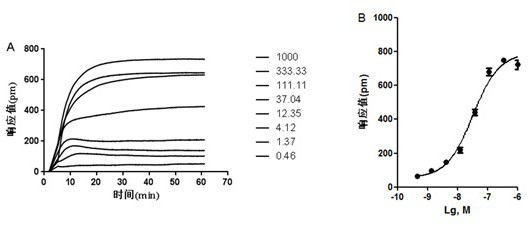
[0058] First, the first step is to obtain the agonist DMR characteristic signal spectrum of the agonist carbachol on HEK-293T-M3 cells:
[0059] Materials Carbachol and tiotropium bromide were purchased from Sigma; refenacin and glycopyrrolate were purchased from Abmole; ipratropium bromide was purchased from Coolaber; human embryonic kidney cells HEK-293T-M3 cells were derived from University of California, Irvine. The detection platform is the third generation of Corning The signal detected by the imager is the wavelength shift caused by the dynamic mass reset (DMR) of the cell and the change value of the resonance wavelength caused by the drug acting on the cell.
[0060] HEK-293T-M3 cells in the logarithmic growth phase were inoculated in a cell-compatible 384 microwell plate, the medium used was DMEM (#SH30022.01B, Thermo), the inoculation volume of each well was 40 μL, and each well was inoculated The number of cells is 2.0 x 10 4 Firstly, place the inoculated cell p...
Embodiment 2
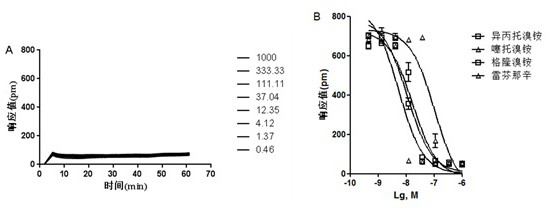
[0066] Based on the antagonistic DMR characteristic signal spectrum of the antagonist refenacin and ipratropium bromide obtained in Example 1 on HEK-293T-M3 cells, the calculated IC50 values are 101.39±30.24nM and 10.81±2.09nM respectively .
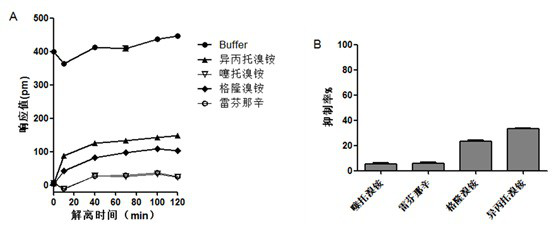
[0067] Then the long-term analysis of the four M3 receptor antagonists:
[0068]HEK-293T-M3 cells in the logarithmic growth phase were inoculated in a cell-compatible 384 microwell plate, the medium used was DMEM (#SH30022.01B, Thermo), the inoculation volume of each well was 40 μL, and each well was inoculated The number of cells was 2.0×104, and the inoculated cell plate was placed in a cell incubator and cultured for 20-22 hours until the cell confluence reached about 95%, and the activity experiment was carried out. Replace the cell culture solution in the microwell plate with Hank's balanced salt solution (containing 10mM HEPES), and add a volume of 30 μL to each well. After adding, place in Equilibrate on the imager for 1 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com