Preparation method of electrocatalyst based on intramolecular or intermolecular asymmetric organic molecules and application of electrocatalyst in zinc-air battery
An electrocatalyst and organic molecule technology, applied in the application field of zinc-air batteries, can solve the problems that the catalytic active sites cannot be precisely synthesized and regulated, and the material system with high catalytic activity cannot be further accurately designed, so as to avoid high cost, The effect of modulating catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of compound 2:
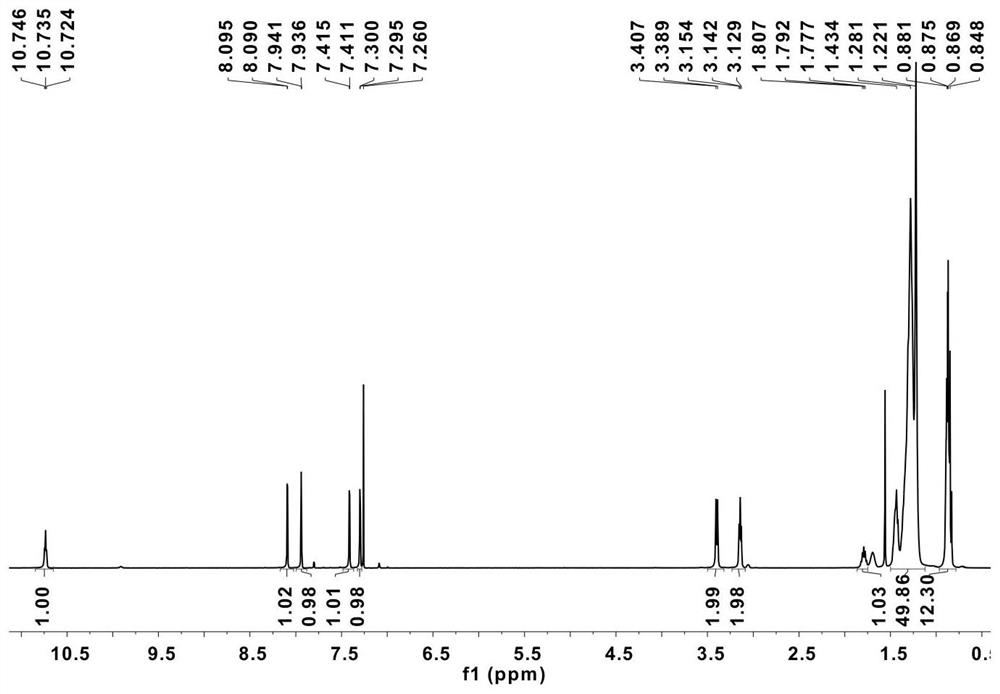
[0067] Under the protection of argon, 800.2mg of compound 1 (reference reference for the preparation method: Polymer AcceptorBased on Double B←N Bridged Bipyridine (BNBP) Unit for High-Efficiency All-Polymer Solar Cells.Adv.Mater, 2016,28(30) :6504-6508; Electron-DeficientBuilding Block Based on B←N Unit for Polymer Acceptor ofAll-Polymer SolarCells.Angew.Chem.Int.Ed.2016,55(4):1436-1440) dissolved in dry dichloromethane ( 15 mL), while slowly adding 10 times equivalents of boron trifluoride diethyl ether and 5 times equivalents of triethylamine dropwise, refluxed at 50°C for 2 hours, cooled to room temperature, distilled off the solvent, extracted the organic phase with n-hexane, Column chromatography separation (dichloromethane: petroleum ether mobile phase) gave compound 2, yield: 570 mg (67%). 1 H NMR (400MHz, CDCl 3 ,20℃): δ10.75-10.72(m,1H),8.09(d,J=2.0Hz,1H),7.94(d,J=2.0Hz,1H),7.41(d,J=1.6Hz, 1H ),7.30(d,J=2.0Hz,1H),3.39(d,J=7.2Hz,...
Embodiment 2
[0079] Compound 4:
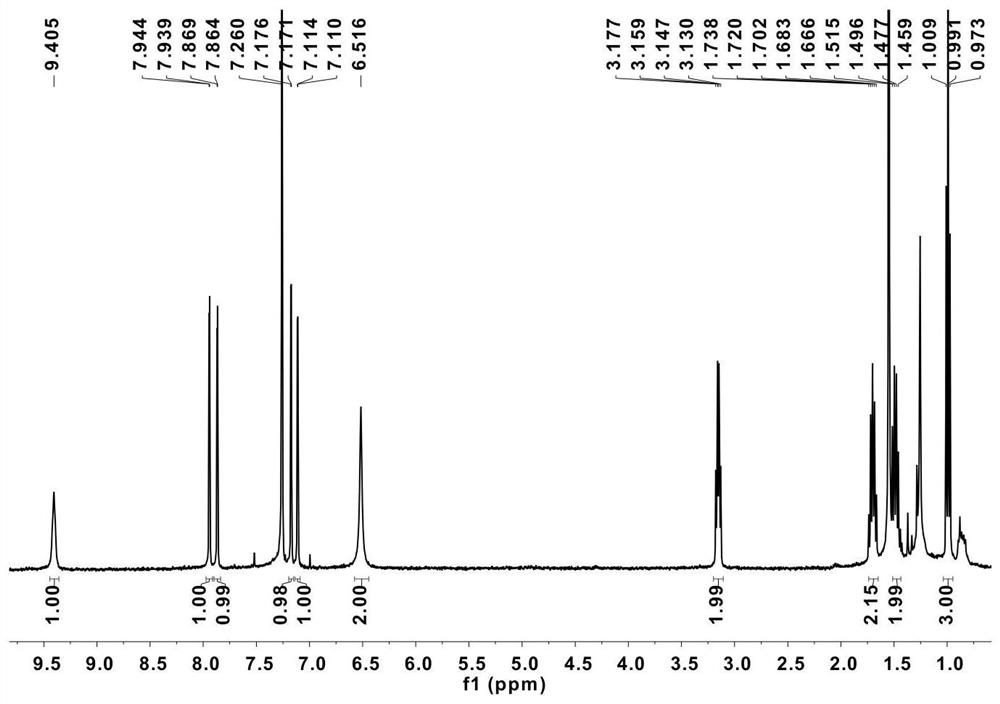
[0080] Dissolve 400.3 mg of compound 3 and 2 equivalents of NaH in dry THF (10 mL), and after reflux at 70°C for 2 h, add 3 equivalents of C 4 h 9 Br, continued to reflux at 70°C for 24h, cooled to room temperature and added a small amount of water dropwise to quench the reaction, extracted with dichloromethane (150mL), and separated by column chromatography (dichloromethane:petroleum ether mobile phase) to obtain compound 4, Yield: 241 mg (52%). 1 HNMR (400MHz, CDCl 3 ,20℃):δ9.41(s,1H),7.94 (d,J=2.0Hz,1H),7.86(d,J=2.0Hz,1H),7.17(d,J=2.0Hz,1H), 7.11(d,J=1.6Hz,1H), 6.52(s,2H),3.18-3.13(m,2H),1.74-1.67(m,2H),1.52-1.46(m,2H),1.01-0.97( m,3H).
[0081]
[0082] Compound 5:
[0083] Dissolve 210.4 mg of compound 4 and 4 equivalents of NaH in dry THF (10 mL), reflux at 70°C for 2 h, then add 4 equivalents of C 16 h 33 1, after continuing to reflux at 70°C for 24h, cool to room temperature and drop a small amount of water to quench the reaction, extrac...
Embodiment 3
[0095] as-BNT:
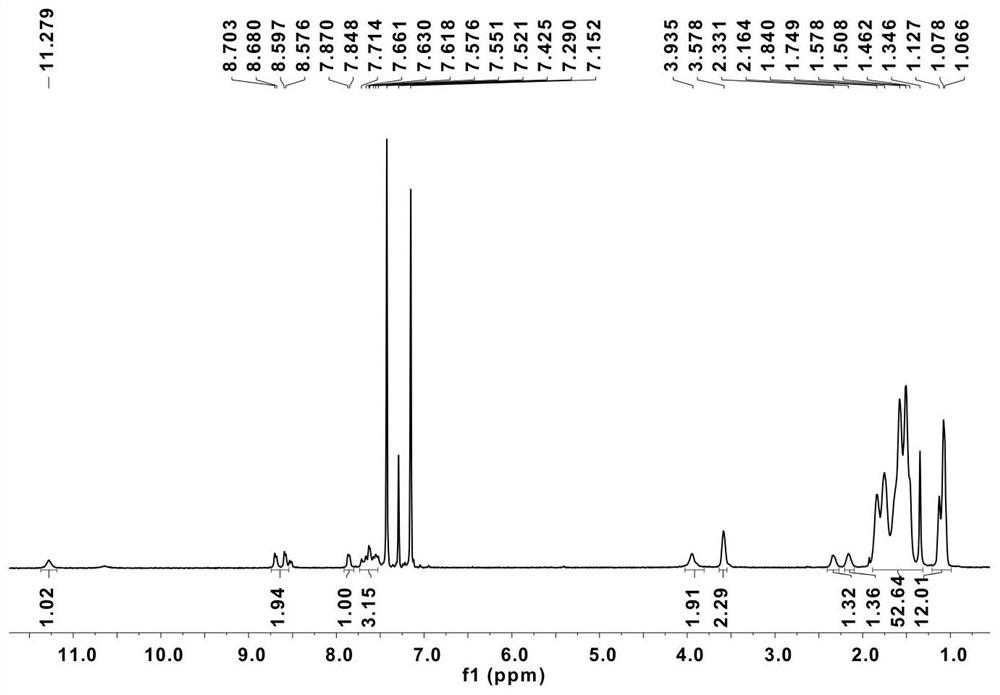
[0096] Under argon protection, 200.2 mg of compound 9, 158.9 mg of 2-tributylstannylthiophene, 0.02 equivalents of tris(dibenzylideneacetone) dipalladium and 0.16 equivalents of tris(o-methylphenyl)phosphorus were dissolved in Dry toluene (20 mL). Refluxed at 100°C for 24h, cooled to room temperature, extracted with dichloromethane (150mL), and separated by column chromatography (dichloromethane:petroleum ether mobile phase) to obtain as-BNT, yield: 196mg (97%). 1 H NMR (400MHz, CDCl 3 ,20℃): δ8.41(d,J=1.6Hz,1H),8.17-8.16(m,1H),7.68(d,J=1.2Hz,1H),7.58-7.48(m,4H), 7.21 -7.18(m,1H),3.59-3.53(m,4H),1.71(d,J=6Hz,2H),1.45-1.25(m,16H),0.97-0.86(m,12H).
[0097]
[0098] In order to explore the advantages of small molecules with asymmetric structure in various properties, we synthesized the corresponding small molecules with symmetrical structure according to the same method. The synthetic route is as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com