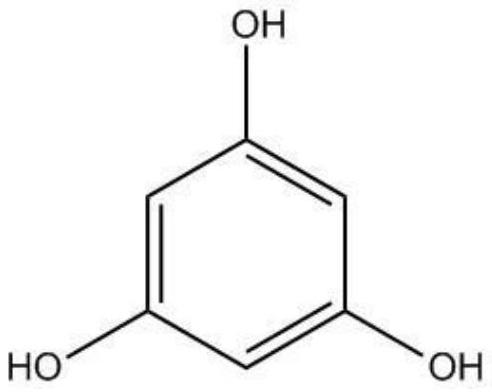
Phloroglucinol injection solution and preparation method thereof
A technology of phloroglucinol injection and phloroglucinol, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor stability and crystallization, and achieve stable High stability, good stability, and the effect of avoiding content loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
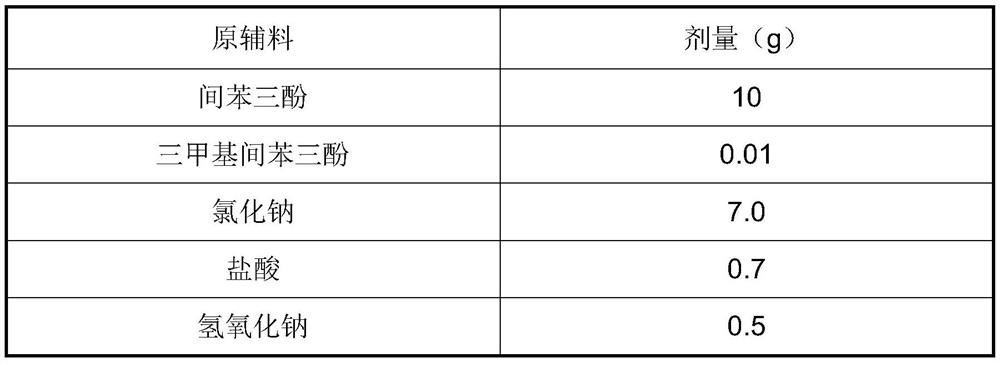
[0041] Raw materials Dose (g) Phloroglucinol 10 Trimethylphloroglucinol 0.01 Sodium chloride 7.0 hydrochloric acid 0.7 sodium hydroxide 0.5 Water for Injection up to 1000ml
[0042] Preparation Process:
[0043] (1) Measure 100ml of water for injection and add it to the airtight liquid mixing container A, pour medical clean nitrogen gas to the liquid level to saturate the water for injection, add sodium hydroxide solution dropwise to adjust the pH to 7.5, mix well, add trimethyl m-benzene Triphenol is stirred and dissolved;
[0044] (2) Add sodium chloride and stir to dissolve in the above-mentioned solution;
[0045] (3) Measure 800ml of water for injection and add it to the airtight dosing container B, add hydrochloric acid solution dropwise and mix well, adjust the pH value of the solution to 3.80, pour medical clean nitrogen gas under the liquid surface to saturate the water for injection, add phloroglucinol Stir to dis...
Embodiment 2
[0051]
[0052]
[0053] Preparation Process:
[0054] (1) Measure 80ml of water for injection and add it to the airtight liquid preparation container A, flush medical clean nitrogen gas to the liquid level to saturate the water for injection, add dropwise sodium hydroxide solution to adjust the pH to 8.0, mix well, add trimethyl m-benzene Triphenol is stirred and dissolved;
[0055] (2) Add sodium chloride and stir to dissolve in the above-mentioned solution;
[0056] (3) Measure 800ml of water for injection and add it to the airtight dosing container B, add hydrochloric acid solution dropwise and mix well, adjust the pH value of the solution to 3.80, pour medical clean nitrogen gas under the liquid surface to saturate the water for injection, add phloroglucinol Stir to dissolve;
[0057] (4) Use fixed-speed pumping devices (peristaltic pumps, etc.) at 20rpm to add the liquids in containers A and B to the dilute container C at a constant rate of 1:10 (protected with m...
Embodiment 3
[0062] Raw materials Dose (g) Phloroglucinol 10 Trimethylphloroglucinol 0.01 Sodium chloride 7.0 hydrochloric acid 0.7 sodium hydroxide 0.5 Water for Injection up to 1000ml
[0063] Preparation Process:
[0064] (1) Measure 70ml of water for injection and add it to the airtight liquid preparation container A, flush medical clean nitrogen gas to the liquid level to saturate the water for injection, add sodium hydroxide solution dropwise to adjust the pH to 7.8, mix well, add trimethyl m-benzene Triphenol is stirred and dissolved;
[0065] (2) Add sodium chloride and stir to dissolve in the above-mentioned solution;
[0066] (3) Measure 840ml of water for injection and add it to the airtight liquid mixing container B, add hydrochloric acid solution dropwise and mix evenly, adjust the pH value of the solution to 3.80, flush medical clean nitrogen to the liquid level to saturate the water for injection, add phloroglucinol Stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com