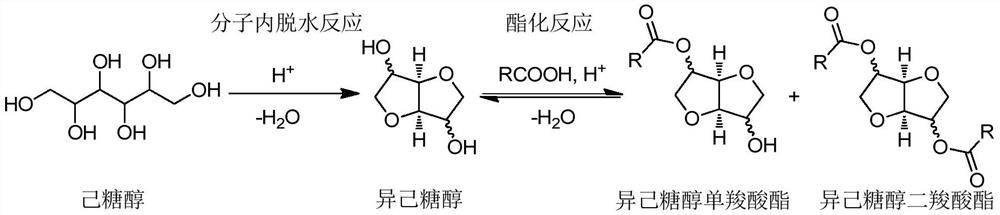
Method for synthesizing isohexitol ester
A technology for isohexitol ester and hexitol, applied in the field of preparing isohexitol ester, can solve the problems of complex operation process, high equipment requirements, long reaction time, etc., and achieves simplified reaction equipment, low equipment requirements, and shortened reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
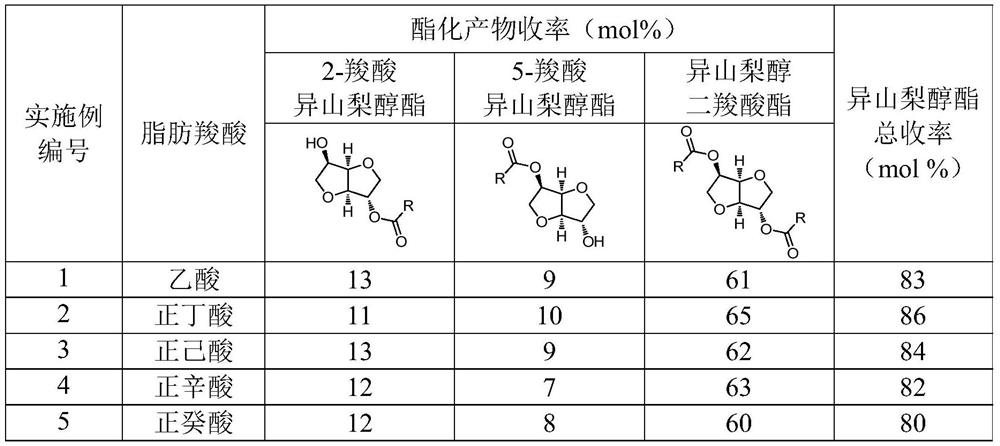
[0044] Different aliphatic carboxylic acids shown in sorbitol, table 1, toluene and solid acid catalyst phosphotungstic heteropolyacid (H 3 PW 12 o 40 ) into the reactor, nitrogen replacement, closed reactor, 170 ℃ magnetic stirring reaction for 6 hours, wherein, the mol ratio of fatty carboxylic acid and sorbitol is 20:1, and the mol ratio of toluene and sorbitol is 10:1, The mass ratio of phosphotungstic heteropoly acid to sorbitol is 0.3:1. After the reaction was completed, the esterification product was quantitatively analyzed by gas chromatography internal standard method, expressed in mole percent (mol%).
[0045] The reaction results are shown in Table 1.
[0046] Table 1: Preparation of Isosorbide Carboxylate by One-Step Conversion of Sorbitol Catalyzed by Phosphotungstic Heteropoly Acid
[0047]
[0048] Typically, solid acid catalyzed sorbitol in aliphatic carboxylic acid one-step conversion of the resulting esterification products are 2-carboxylate isosorbide...
Embodiment 6
[0050] Put sorbitol, acetic acid, diethyl ketone and solid acid catalyst H-ZSM-5 molecular sieve into the reactor, replace with nitrogen, seal the reactor, and react with magnetic stirring at 190°C for 4 hours. Wherein, the molar ratio of acetic acid to sorbitol is 40:1, the molar ratio of diethyl ketone to sorbitol is 10:1, and the mass ratio of H-ZSM-5 molecular sieve to sorbitol is 0.2:1. Treat that reaction finishes, adopt gas chromatography internal standard method to carry out quantitative analysis to esterification product, represent with mole percentage (mol%), gained 2-acetate isosorbide yield is 13mol%, 5-acetate isosorbide yield 7mol%, the yield of isosorbide diacetate is 62mol%, and the total yield of isosorbide is 82mol%.
Embodiment 7
[0052] Put sorbitol, butyric acid, methyl isopropyl ketone and solid acid catalyst H-beta molecular sieve into the reactor, replace with nitrogen, seal the reactor, and react with magnetic stirring at 180°C for 10 hours. Wherein, the mol ratio of butyric acid and sorbitol is 4:1, the mol ratio of methyl isopropyl ketone and sorbitol is 4:1, and the mass ratio of H-beta molecular sieve and sorbitol is 0.01:1. Treat that reaction finishes, adopt gas chromatography internal standard method to carry out quantitative analysis to esterification product, represent with mole percentage (mol%), gained 2-butyric acid isosorbide yield is 11mol%, 5-butyric acid isosorbide The yield is 9 mol%, the yield of isosorbide dibutyrate is 65 mol%, and the total yield of isosorbide is 85 mol%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com