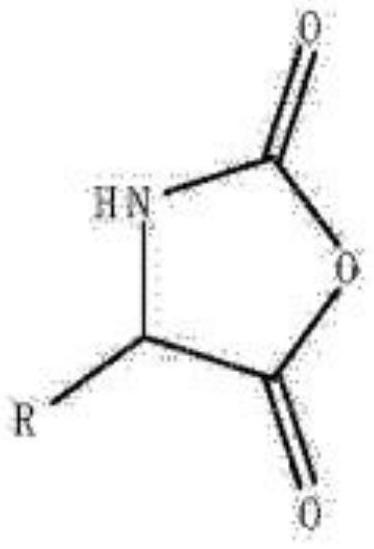
Method for measuring chloride ions in amino acid-N-formic anhydride
A determination method and technology of chloride ions, which are applied in the direction of color/spectral characteristic measurement, etc., can solve the problems of expensive ion chromatography, harsh operation, and the need for organic solvents, and achieve the effects of reducing equipment weight, reducing workload, and improving body strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Accurately weigh 0.165g of sodium chloride to a 1000ml measuring bottle, add an appropriate amount of water to dissolve and dilute to the mark, shake well, and use it as a stock solution; accurately measure 10ml of the stock solution to a 100ml measuring bottle, add water to dilute to the mark, and shake well to obtain ( Every 1ml is relative to the Cl) sodium chloride standard solution of 10ug.
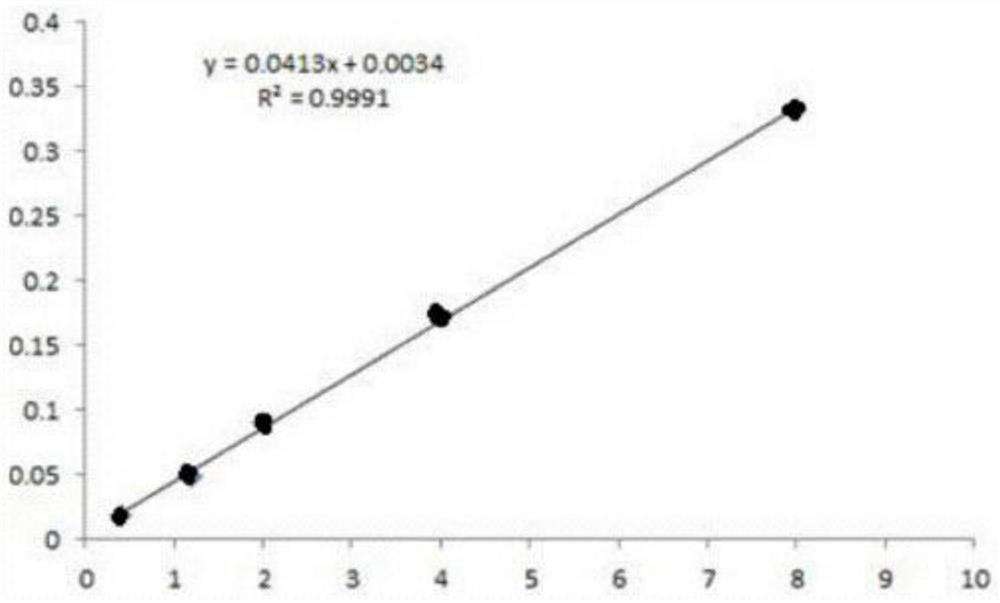
[0037] Accurately measure NaCl standard solution (1ml, 3ml, 5ml) and stock solution (1ml, 2ml) respectively to a 25ml measuring bottle, add 5% NaOH 10ml, add nitric acid 2ml, 0.1mol / L silver nitrate 1ml, add water to volume. After standing in the dark for 0.5 hours, measure the absorbance at 440nm, and make a working curve.
[0038] Table I
[0039] Chloride ion concentration (ug / ml) Absorbance 0.4 0.018 1.2 0.049 2 0.091 4 0.172 8 0.332
Embodiment 2
[0041] Repeat Example 1 (in order to obtain the working curve of the external standard method).
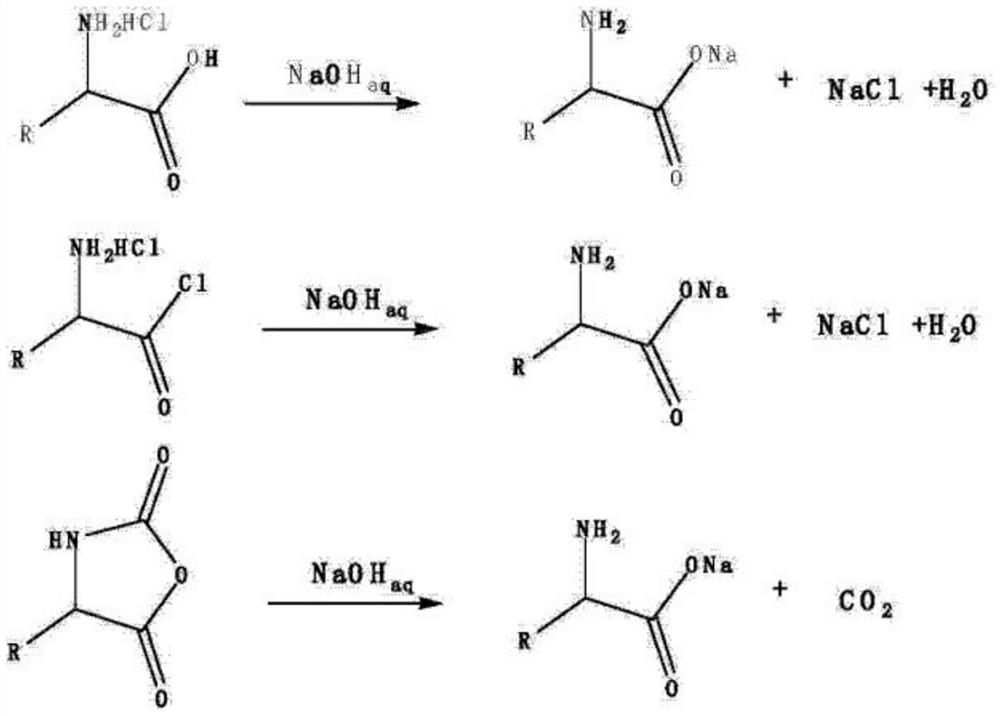
[0042] The 500mg aspartic acid-NCA that accurately weighs is transferred in the round bottom flask of 100ml, adds the alkali solution of 40ml 5%NaOH (wt), 100 ℃ of heating and stirring for half an hour, after cooling to room temperature, the reaction solution is transferred to In a 100ml volumetric flask, add 15ml of concentrated nitric acid to adjust the pH to acidic, then add 5ml of 0.1NAgNO3, react at 25°C for 0.5 hours, and then add deionized water to make it volume. Measure the absorbance of the solution under the condition of 440nm, and conduct a blank test at the same time.
Embodiment 3
[0044] Repeat Example 1 (in order to obtain the working curve of the external standard method).
[0045] Accurately weighed 500mgL-aspartic acid-4-benzyl ester-N-carbonyl anhydride is transferred to a 100ml round-bottomed flask, and 40ml of 5%NaOH (wt) alkali solution is added, heated and stirred at 25°C for half an hour, and the Transfer the reaction solution to a 100ml volumetric flask, add 15ml of concentrated nitric acid to adjust the pH to acidic, then add 5ml of 0.1NAgNO3, react at 25°C for 0.5 hours, and then add deionized water to make it volume. Measure the absorbance of the solution under the condition of 440nm, and conduct a blank test at the same time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com