Sulfide solid electrolyte and preparation method and application thereof
A technology of solid electrolyte and sulfide, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of low conductivity of sulfide solid electrolytes, eliminate the decline in ion transmission capacity, improve ion conductivity, and conduct electricity. rate-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
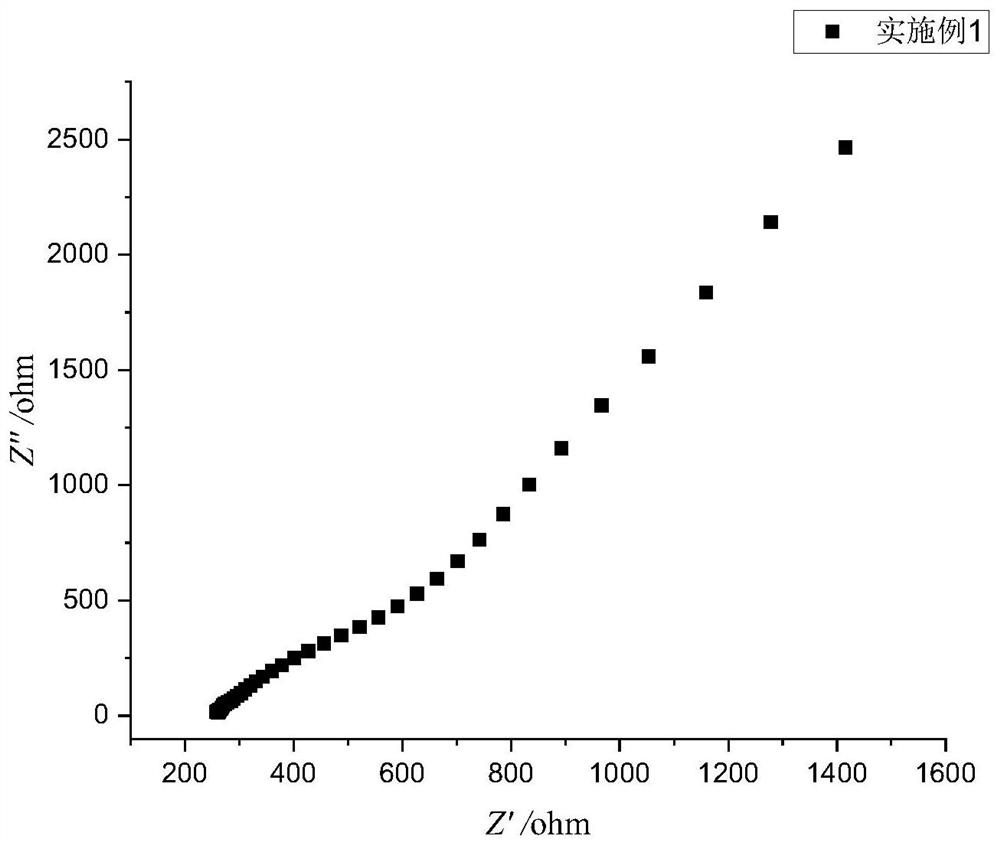
Embodiment 1
[0050] A preparation method of a sulfide solid electrolyte, the preparation steps are as follows:
[0051] (1) In a glove box under an oxygen-free and water-free argon atmosphere, weigh Li at a mass ratio of 75:25 2 S and P 2 S 5 , were prepared by dissolving with anhydrous acetonitrile to prepare a solution, respectively, to obtain Li with a concentration of 0.05mol / L 2 S solution and P with a concentration of 0.0167mol / L 2 S 5 solution; the mass is Li 2 S and P 2 S 5 1% of the total mass of aluminum nitrate and mass of Li 2 S and P 2 S 5 0.83% of the total mass of ammonium fluoride was dissolved in absolute ethanol to prepare a solution, respectively, to obtain an aluminum nitrate solution with a concentration of 0.01mol / L and an ammonium fluoride solution with a concentration of 0.03mol / L;
[0052] (2) Under an oxygen-free and water-free argon atmosphere, Li 2 Add the S solution into the high-pressure stirred tank, and then add the P 2 S 5 The solution was adde...
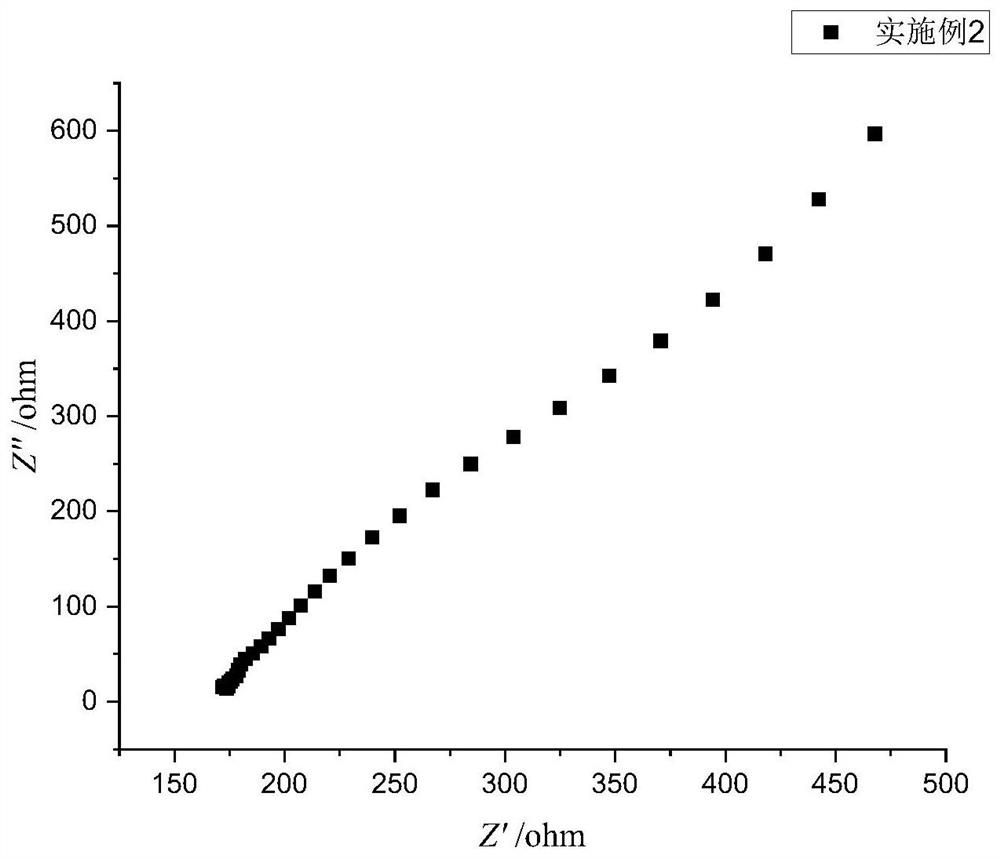
Embodiment 2
[0058] A preparation method of a sulfide solid electrolyte, the preparation steps are as follows:
[0059] (1) In a glove box under an oxygen-free and water-free argon atmosphere, weigh Li at a mass ratio of 70:30 2 S and P 2 S 5 ; Weighing Li 2 S and P 2 S 5 The total mass of 2% LiBH was dissolved in anhydrous tetrahydrofuran to form a solution respectively to obtain LiBH with a concentration of 0.06mol / L. 2 S solution, to obtain P with a concentration of 0.0257mol / L 2 S 5 solution and 0.05mol / L LiBH solution; the mass will be Li 2 S and P 2 S 5 1.2% of the total mass of aluminum nitrate and mass of Li 2 S and P 2 S 5 0.995% of the total mass of ammonium fluoride was dissolved in absolute ethanol to prepare a solution respectively to obtain an aluminum nitrate solution with a concentration of 0.012mol / L and an ammonium fluoride solution with a concentration of 0.036mol / L;
[0060] (2) Under an oxygen-free and water-free argon atmosphere, Li 2 Add the S solution ...
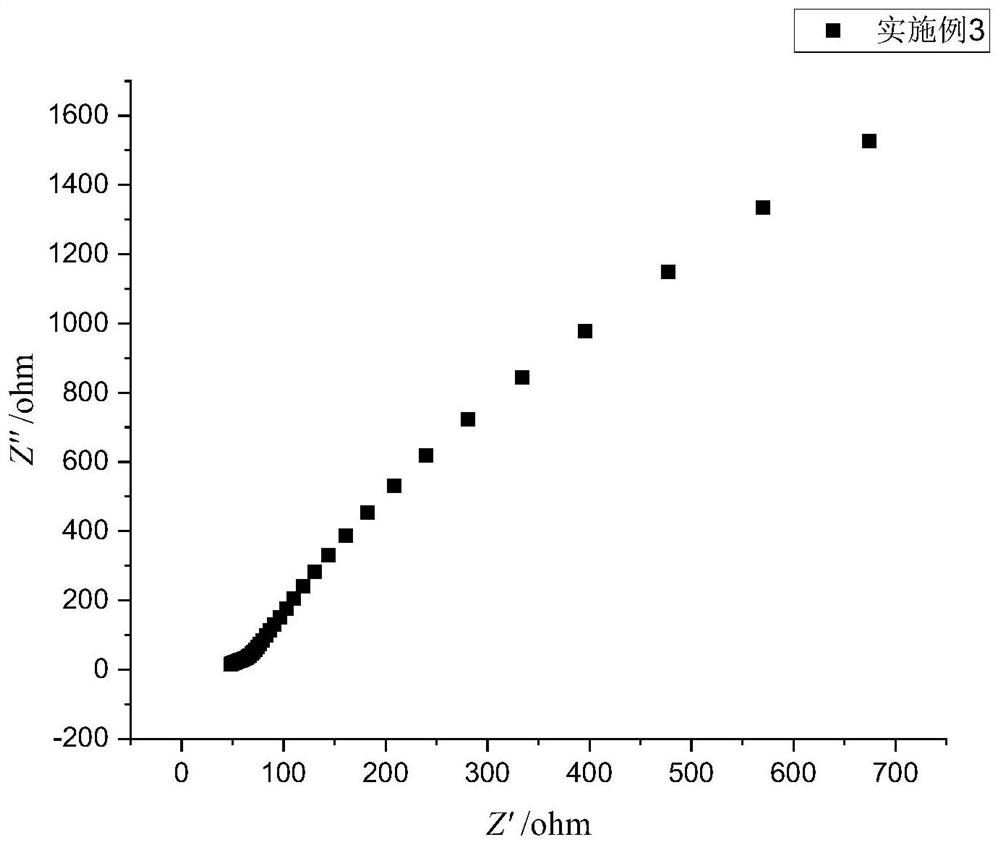
Embodiment 3
[0066] A preparation method of a sulfide solid electrolyte, the preparation steps are as follows:
[0067] (1) In a glove box under an oxygen-free and water-free argon atmosphere, weigh Li at a mass ratio of 90:10 2 S and P 2 S 5 ; Weighing Li 2 S and P 2 S 5 Total mass 35% SnS 2 , were prepared by dissolving with anhydrous acetonitrile to form a solution, respectively, to obtain Li with a concentration of 0.072mol / L 2 S solution, P with a concentration of 0.008mol / L 2 S 5 solution and 0.01mol / L SnS 2 solution; the mass is Li 2 S and P 2 S 5 1.5% of the total mass of aluminum nitrate and mass of Li 2 S and P 2 S 5 1.25% of the total mass of ammonium fluoride was dissolved in anhydrous methanol to prepare a solution, respectively, to obtain an aluminum nitrate solution with a concentration of 0.015mol / L and an ammonium fluoride solution with a concentration of 0.045mol / L;
[0068] (2) Under an oxygen-free and water-free argon atmosphere, Li 2 Add the S solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com