Dialkyl phosphinate compound containing active epoxy group as well as preparation and application thereof
A technology of dialkyl phosphinic acid and epoxy group, applied in the field of dialkyl phosphinic acid esters, can solve problems such as inapplicability, and achieve the effect of reducing dosage and good flame retardant performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 has the compound preparation in the structure in formula (III)
[0058]
[0059] Add 288g (2mol) sodium diethylphosphinate, 6.0g catalyst polyalkylammonium bromide, 600g epichlorohydrin in the autoclave that stirrer, thermometer are housed, open and stir, pass into nitrogen replacement three times, Raise the temperature to 110-120°C, heat and stir for 10 hours, and then turn on the vacuum to remove unreacted epichlorohydrin. The product was filtered and purified by distillation to obtain 338 g of liquid product with a conversion rate of 95%.
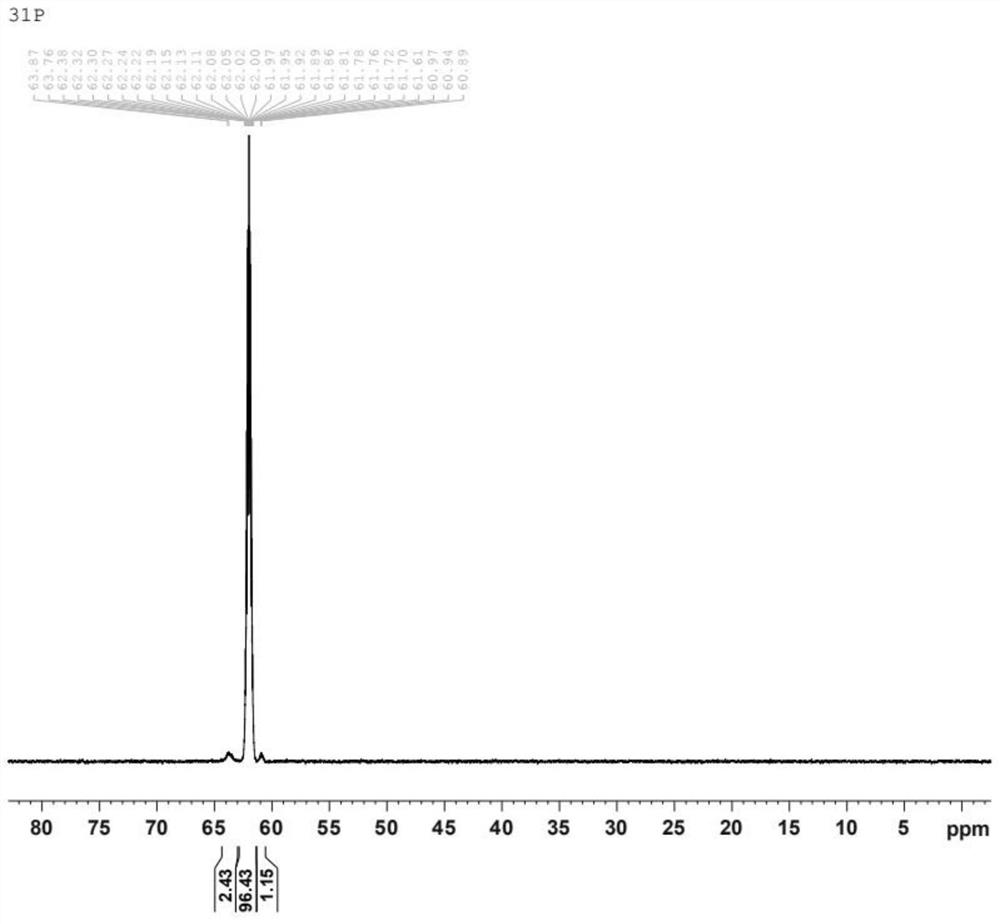
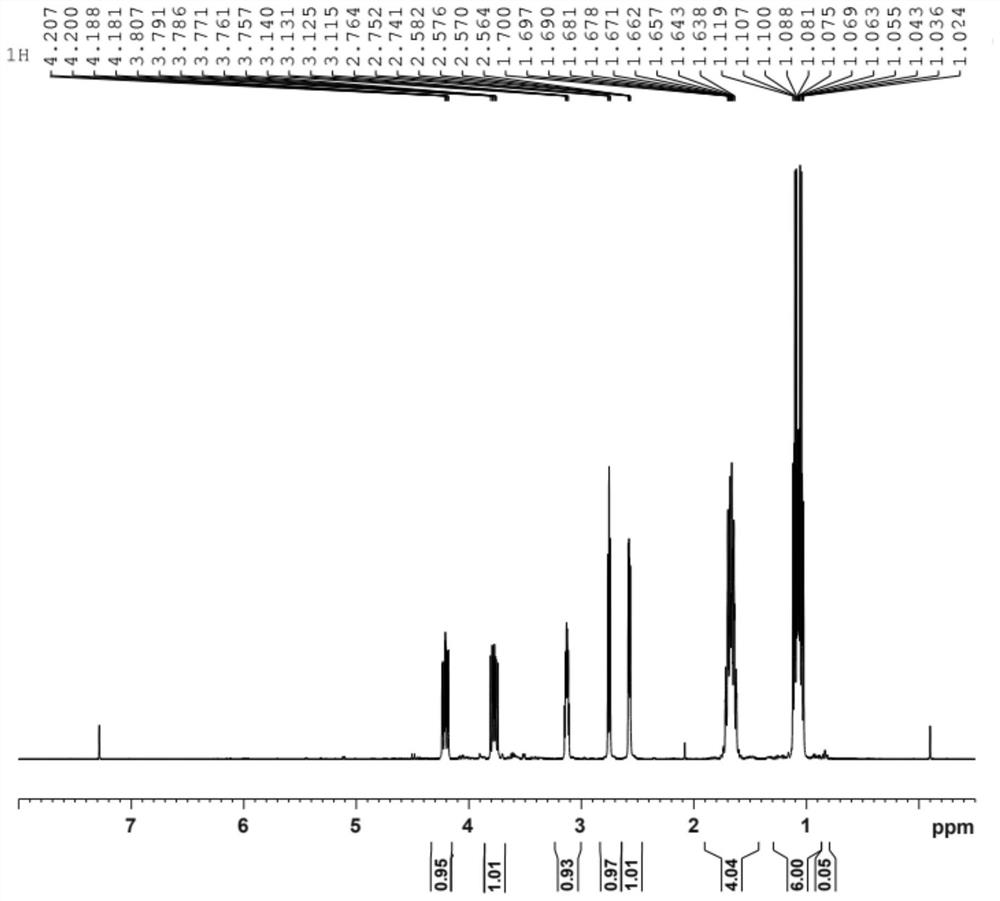
[0060] Characterize by carrying out NMR-P spectrum and NMR-H spectrum to obtained product, the result is as attached figure 1 (phosphorus NMR) and attached figure 2 (Proton NMR). through with image 3 (Sodium diethyl hypophosphite phosphorus NMR) comparison can be known, in figure 1 Among them, the peak between the offset 60-65ppm represents the structure of diethyl hypophosphite, and the newly synthesized co...
Embodiment 2
[0062] Embodiment 2 prepares flame-retardant epoxy material
[0063] 1) the reaction of formula (III) compound and DDS
[0064] Mix the compound of formula (III) prepared in Example 1 and the epoxy resin curing agent DDS uniformly in a certain proportion, take a small amount of samples and test the DSC curve, and judge the reaction between the compound of formula (III) and DDS according to the exothermic peak in the DSC curve. The DSC curve obtained from the test is as follows Image 6 shown.
[0065] From the results, the DSC curve of DDS alone shows that DDS has a melting point peak at 179.3°C due to being a solid, which is an endothermic process; The exothermic peak indicates that the compound of structure (III) undergoes a ring-opening reaction and releases heat; on the DSC curve of the compound of structure (III) and DDS, there are two exothermic peaks, namely 210.0°C and 264.1°C Compared with the exothermic peak of the single structure (III) compound, the peaks are ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com