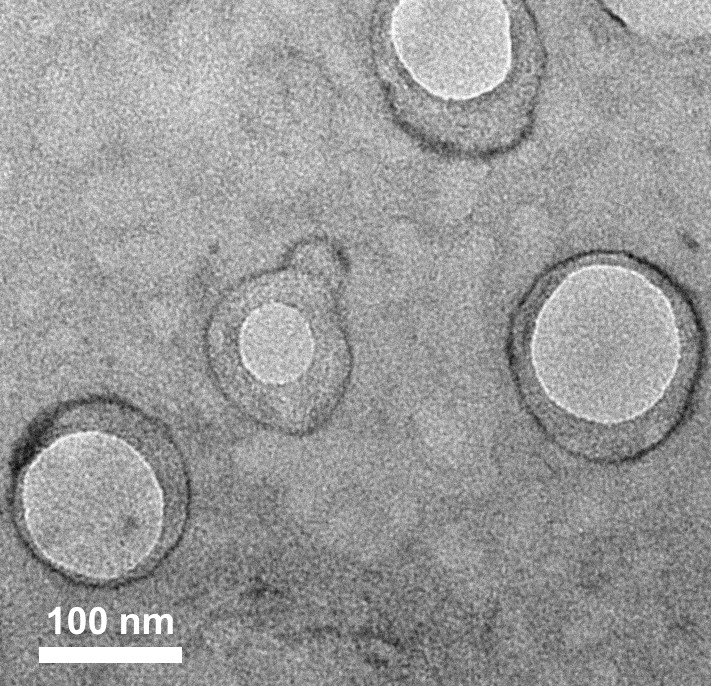
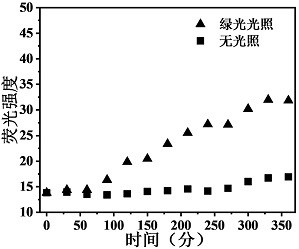
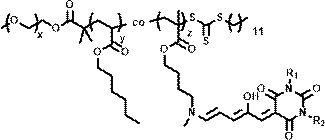
Green light response polymer nano-drug carrier and preparation method thereof
A nano-drug carrier and polymer technology, applied in the field of biomedical materials, can solve problems that limit the clinical application of polymer nano-drug carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032](1) Preparation of furan derivatives: 1.51 g is acid or Babi acid and 0.961 g of furfural is dissolved in 30 ml of water, and the reaction is 16 h at room temperature; after completion of the completion, it is filtered, water washing and other means to obtain a yellow solid. The yellow solid was redissolved in dichloromethane and washed sequentially with saturated sodium hydrogen sulfite solution, ultra-pure water, saturated sodium carbonate solution and saturated sodium chloride solution. Finally, dried over anhydrous magnesium sulfate Waiting for the means to obtain furan derivatives;
[0033](2) Polyethylene glycol -b- Poly (Acrylate -CO - Preparation of pentafluorophenyl methacrylate: 1 part of 2- (dodecyl trikarcarbonate group) -2-methylpropylene polyethylene glycol monomethyl ether, 0.05 parts of two Isobutyronitrile, 4 parts of methacrylate, 40 parts of acrylate, hexyl acrylate, dissolved in 1 ml dioxane, 60 ° C reaction 2 h; after the reaction is completed, the means of d...
Embodiment 2
[0038](1) Preparation of furan derivatives: 1.51 g is acid or Babi acid and 0.961 g of furfural is dissolved in 30 ml of water, and the reaction is 16 h at room temperature; after completion of the completion, it is filtered, water washing and other means to obtain a yellow solid. The yellow solid was redissolved in dichloromethane and washed sequentially with saturated sodium hydrogen sulfite solution, ultra-pure water, saturated sodium carbonate solution and saturated sodium chloride solution. Finally, dried over anhydrous magnesium sulfate Waiting for the means to obtain furan derivatives;
[0039](2) Polyethylene glycol -b- Poly (Acrylate -CO - Preparation of pentafluorophenyl methacrylate: 5 parts of 2- (dodecyl trikarcarbonate) -2-methacol polyethylene glycol monomethyl ether, 0.1 parts Isobutyronitrile, 5 parts of methacrylate, 60 parts of acrylate (adherehex) dissolved in 5 ml dioxane, 15 h at 75 ° C; after the reaction is completed, the polyethylene glycol is treated by dialys...
Embodiment 3
[0044](1) Preparation of furan derivatives: 1.51 g is acid or Babi acid and 0.961 g of furfural is dissolved in 30 ml of water, and the reaction is 16 h at room temperature; after completion of the completion, it is filtered, water washing and other means to obtain a yellow solid. The yellow solid was redissolved in dichloromethane and washed sequentially with saturated sodium hydrogen sulfite solution, ultra-pure water, saturated sodium carbonate solution and saturated sodium chloride solution. Finally, dried over anhydrous magnesium sulfate Waiting for the means to obtain furan derivatives;
[0045](2) Polyethylene glycol -b- Poly (Acrylate -CO - Preparation of pentafluorophenyl methacrylate: 10 parts of 2- (dodecyl trikarcarbonate) -2-methylpropylene polyethylene glycol monomethyl ether, 0.2 parts Isobutyronitrile, 8 parts of methacrylate, 80 parts of acrylate (adherene) dissolved in 10 ml dioxane, 90 ° C reaction 30 h; after completion, treatment, polyethylene glycol -b- Poly (Acry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com