PCV3 Cap protein epitope peptide, anti-PCV3 Cap protein monoclonal antibody, and preparation method and application thereof
A monoclonal antibody and protein technology, applied in the field of cellular immunity, can solve the problem of few immunological diagnostic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The selection and preparation of embodiment 1 immunogen
[0048] 1. Selection of Immunogen
[0049] The inventors have long-term, a large number of studies, practices, and experiments have found that: first, among the proteins encoded by the PCV3 genome, the capsid protein encoded by ORF2, that is, the Cap protein, is a highly immunogenic protein; secondly, in the body, the Cap protein The induced immune reactivity is much higher than that of other proteins, so it is easy to obtain high-affinity monoclonal antibodies; again, Cap protein is the only structural protein of PCV3, and is the preferred target for PCV3 immunological detection method research and vaccine development.
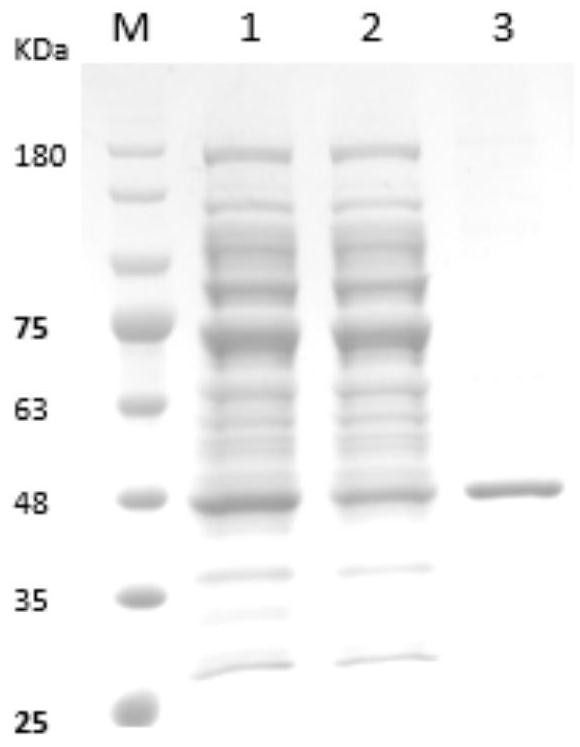
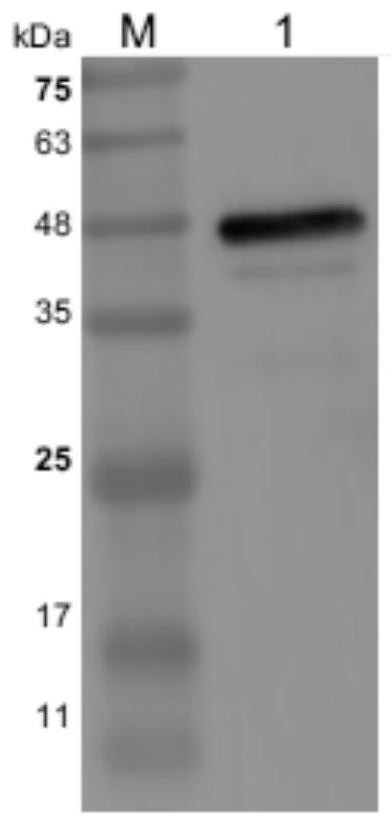
[0050] 2. Preparation of Immunogen
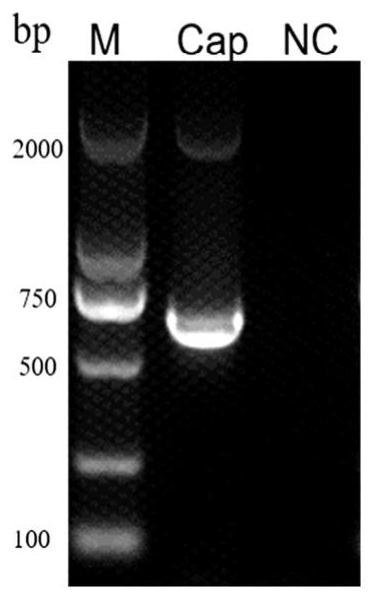
[0051] (1) Construct the pGEX6P-1-Cap recombinant vector expressing Cap protein, and use PCR and sequencing to identify whether the recombinant vector is constructed successfully (identification results are as follows: figure 1 shown);
[0052] (2) Transfor...
Embodiment 2
[0056] Screening and identification of embodiment 2 positive hybridoma cell lines
[0057] 1. Animal immunization
[0058] (1) Add immunogen Cap protein to complete Freund's adjuvant for first immunization;
[0059] (2) Immunize 6 female BALB / c mice aged 4 to 8 weeks by multi-point subcutaneous injection on the back, with an immune dose of 25 μg / mouse;
[0060] (3) BALB / c mice were boosted with the same method and dosage after being emulsified with Freund's incomplete adjuvant and immune antigen every 3 weeks;
[0061] (4) After three times of booster immunization, BALB / c mice were super-immunized with immunogen without adjuvant by tail vein injection 3 to 4 days before cell fusion, and the immunization dose was 50 μg / mouse;
[0062] (5) Determination of titer and sensitivity of multiple antiserum:
[0063] One week after the last booster immunization, tail-cut blood was collected from 6 mice respectively, and then the titers of polyantiserum of 6 mice were measured by indi...
Embodiment 3
[0076] Example 3 Preparation, purification and identification of monoclonal antibody ascites
[0077] 1. Preparation of monoclonal antibody by inducing ascites in vivo
[0078] Select multiparous female Balb / c mice, intraperitoneally inject 500 μl of sterilized paraffin, and a week later, intraperitoneally inject the obtained monoclonal hybridoma cells again, the injection volume is 2×10 5 After another week, the ascites was extracted after the abdomen of the mouse was enlarged, and the supernatant was obtained after centrifugation, and the ascites was purified by octanoic acid ammonium sulfate method.
[0079] 2. Antibody Purification
[0080] Antibody purification by saturated ammonium sulfate precipitation method, the operation method is as follows:
[0081] 1). Take 5ml of monoclonal antibody ascites, add 5ml of PBS buffer solution, and then add 2.5ml of saturated ammonium sulfate solution drop by drop to make a final concentration of 20% ammonium sulfate solution. Stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com