Co-production method of caustic soda and ferric orthophosphate
A technology of ferric orthophosphate and caustic soda, applied in the field of electrolysis, can solve problems such as waste of production capacity, achieve the effect of reducing energy consumption and overcoming excess production capacity of chlorine gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
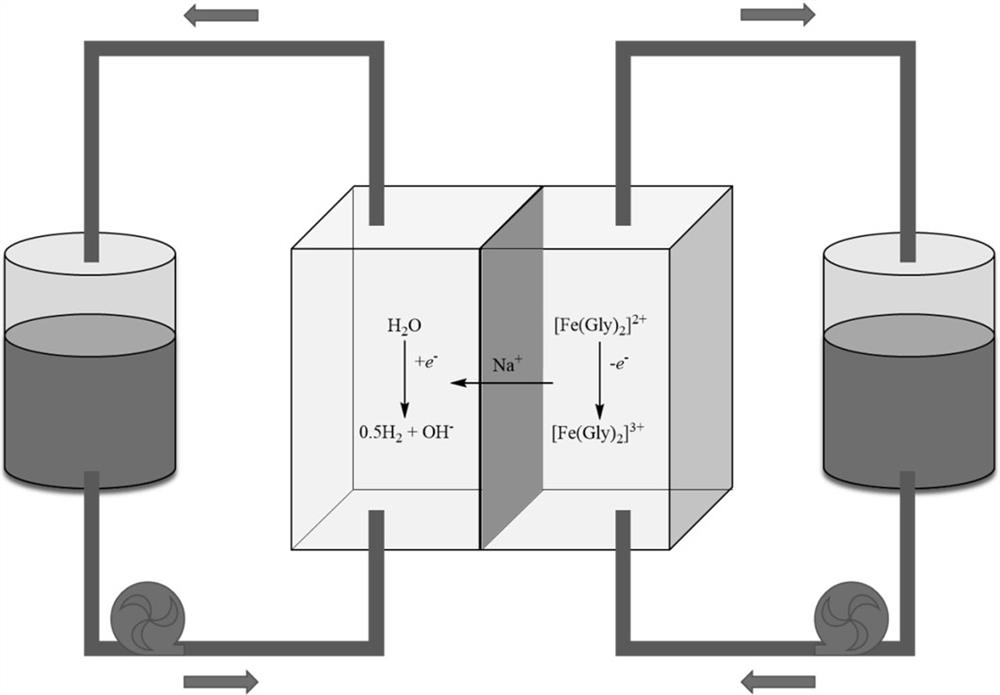
[0025] Dissolve glycine at a concentration of 1 mol / L in 20 mL of 1 mol / L sodium sulfate aqueous solution, and then dissolve ferrous sulfate at a concentration of 0.5 mol / L under stirring to obtain an anolyte. Use 20mL of 1mol / L sodium sulfate aqueous solution as the catholyte. Electrolyte is stored separately in two storage tanks. The preparation of the anode current collector is as follows: a carbon felt with a thickness of 5 mm was fired in air at 400 °C for 24 hours, and then bonded with a graphite plate as the anode current collector. The preparation of the cathode current collector is as follows: a 5 mm thick carbon felt was burned in air at 400 °C for 24 hours, a platinum-coated titanium mesh of the same area was inserted in the middle, and the whole was bonded with a graphite plate as the cathode current collector. In this example, the current collector area is 10 cm 2 . Then, place the 10 cm 2 The Nafion117 membrane was treated in 1 mol / L NaOH at 80 °C for 6 hour...
Embodiment 2
[0027] Dissolve glycine at a concentration of 1 mol / L in 20 mL of 1 mol / L sodium sulfate aqueous solution, and then dissolve ferrous sulfate at a concentration of 0.5 mol / L under stirring to obtain an anolyte. Use 20mL of 1mol / L sodium sulfate aqueous solution as the catholyte. Electrolyte is stored separately in two storage tanks. The preparation of the anode current collector is as follows: a carbon felt with a thickness of 5 mm was fired in air at 400 °C for 24 hours, and then bonded with a graphite plate as the anode current collector. The preparation of the cathode current collector is as follows: burn a 5mm thick carbon felt in air at 400°C for 24 hours, insert a platinum-coated titanium mesh with the same area in the middle, and bond the whole with a graphite plate as the cathode current collector. In this example, the current collector area is 10 cm 2 . Then, place the 10 cm 2 The Nafion117 membrane was treated in 1 mol / L NaOH at 80 °C for 6 hours, used as a batte...
Embodiment 3
[0029] Dissolve glycine at a concentration of 1 mol / L in 20 mL of 2 mol / L sodium chloride aqueous solution, and then dissolve ferrous chloride at a concentration of 0.5 mol / L under stirring to obtain an anolyte. Use 20mL of 1mol / L sodium sulfate aqueous solution as the catholyte. Electrolyte is stored separately in two storage tanks. The preparation of the anode current collector is as follows: a carbon felt with a thickness of 5 mm was fired in air at 400 °C for 24 hours, and then bonded with a graphite plate as the anode current collector. The preparation of the cathode current collector is as follows: a 5 mm thick carbon felt was burned in air at 400 °C for 24 hours, a platinum-coated titanium mesh of the same area was inserted in the middle, and the whole was bonded with a graphite plate as the cathode current collector. In this example, the current collector area is 10 cm 2 . Then, place the 10 cm 2 The Nafion117 membrane was treated in 1 mol / L NaOH at 80 °C for 6 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com