A kind of coproduction method of caustic soda and ferric orthophosphate
A technology of ferric orthophosphate and caustic soda, which is applied in the field of electrolysis, can solve problems such as waste of production capacity, and achieve the effect of reducing energy consumption and overcoming excess production capacity of chlorine gas.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
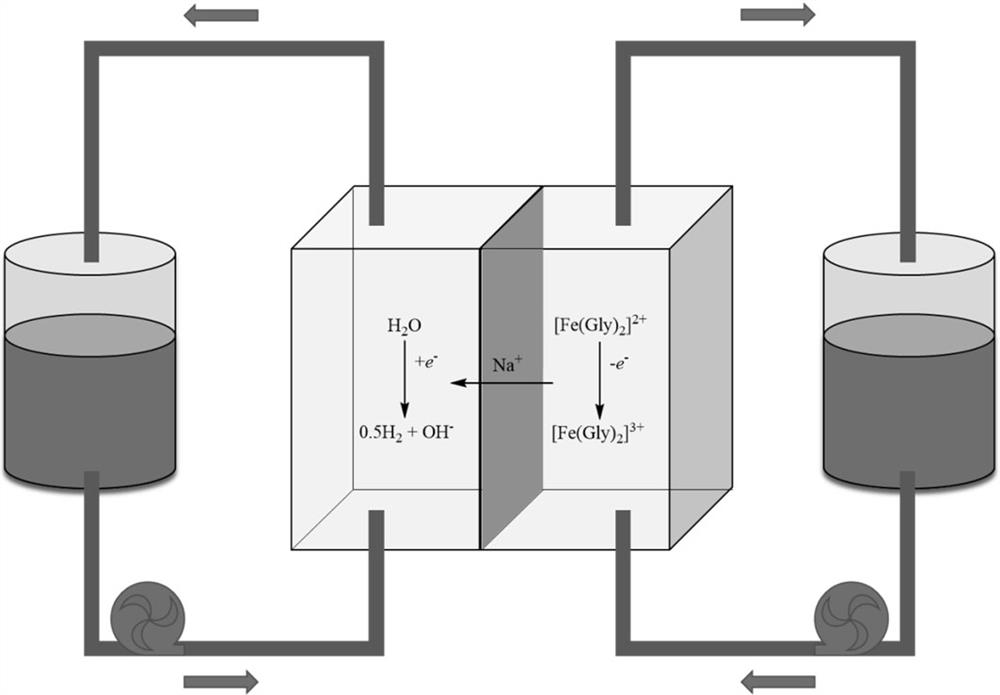
[0025] The glycine was dissolved in an aqueous solution of 20 ml of 1 mol / L of the sodium sulfate solution at a concentration of 1 mol / L, and then the iron sulfate was dissolved according to the concentration of 0.5 mol / L under stirring. Anode electrolyte. The aqueous sodium sulfate solution of 20 ml of 1 mol / L was used as the cathode electrolyte. The electrolyte is stored in two storage tanks, respectively. The preparation of the anode concentration fluid is as follows: 5 mm thick carbon felt baked for 24 hours in air at 400 ° C, and the graphite sheet is combined as an anodic concentration fluid. The prepared by the cathode concentration fluid is followed: 5 mm thick carbon felt baked for 24 hours in 400 ° C air, inserted the mitolating titanium-plated titanium web of the same area, and the entire graphite plate is engaged as a cathode current collector. In this embodiment, the collector area is 10 cm. 2 . Then, 10 cm will be 2 The Nafion117 film was treated at 80 ° C in...
Embodiment 2
[0027] The glycine was dissolved in an aqueous solution of 20 ml of 1 mol / L of the sodium sulfate solution at a concentration of 1 mol / L, and then the iron sulfate was dissolved according to the concentration of 0.5 mol / L under stirring. Anode electrolyte. The aqueous sodium sulfate solution of 20 ml of 1 mol / L was used as the cathode electrolyte. The electrolyte is stored in two storage tanks, respectively. The preparation of the anode concentration fluid is as follows: 5 mm thick carbon felt baked for 24 hours in air at 400 ° C, and the graphite sheet is combined as an anodic concentration fluid. The prepared by the cathode concentration fluid is followed: 5 mm thick carbon felt baked for 24 hours in 400 ° C air, inserted the mitolating titanium-plated titanium web of the same area, and the entire graphite plate is engaged as a cathode current collector. In this embodiment, the collector area is 10 cm. 2 . Then, 10 cm will be 2 The Nafion117 film was treated at 80 ° C in...
Embodiment 3
[0029] Glycine accordance with 1mol / L was dissolved in a concentration of 20mL2mol / L aqueous solution of sodium chloride, ferrous chloride under stirring in accordance with a concentration of 0.5 mol / L was dissolved anolyte obtained. In 20mL1mol / L aqueous sodium sulfate solution as the catholyte. Electrolyte are stored in two tanks. Anode current collector prepared as follows: A 5 mm thick carbon felt burned for 24 hours at 400 deg.] C in air, in cooperation with the graphite plate is attached to the anode current collector. Cathode current collector was prepared as follows: A 5mm thick carbon felt burning 24 hours in air at 400 deg.] C, the same area platinized titanium mesh inserted in the middle, in cooperation with the graphite plate integrally attached to the cathode current collector. In this embodiment, the current collector is 10 cm on the area 2 . Then, 10 cm 2 Nafion117 film for 6 hours in 1 mol / L NaOH in 80 ℃, as a battery separator, assembled into a flow batt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com