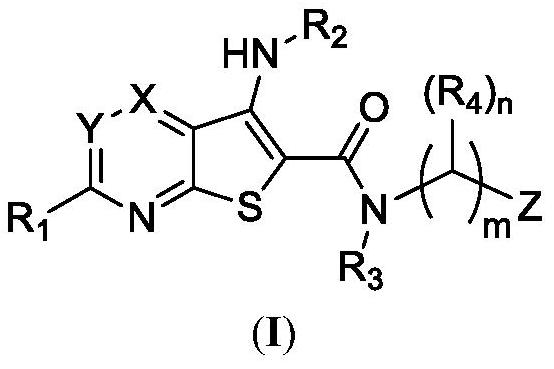
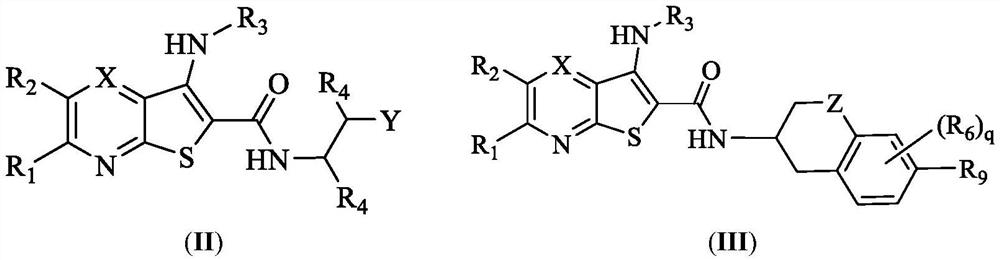
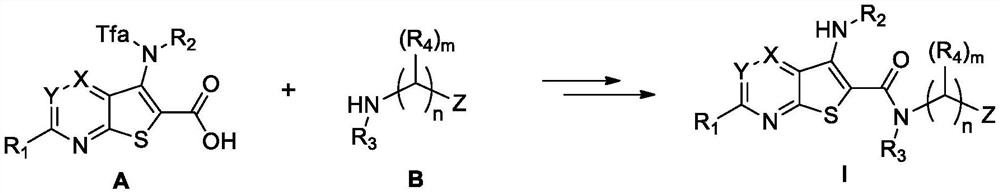
Preparation and application of deubiquitinating enzyme inhibitor
A technology of compounds and nitrogen oxides, applied in anti-inflammatory agents, digestive system, organic chemistry, etc., can solve problems such as tumor cell death, excessive degradation of target proteins, and hindrance to deubiquitination of target proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Synthesis of intermediate tert-butyl 2-acetylthioacetate
[0085] Potassium thioacetate (239.84g, 2.1mol) and anhydrous DMF (500mL) were added to the reaction flask (1L), cooled to 0–5°C, and tert-butyl bromoacetate (390.10g, 2.0mol ), the reaction was continued for 1 h after the dropwise addition at room temperature. The solvent was distilled off from the reaction solution under reduced pressure at 80°C. After cooling, water (500 mL) was added to separate the organic layer. The aqueous layer was extracted with chloroform (150 mL×2), and the combined organic layers were washed with saturated NaCl solution (200 mL×3). Na 2 SO 4 Dry, filter, and concentrate under reduced pressure to obtain tert-butyl 2-acetylthioacetate (380.5 g of red liquid, yield 100%).
[0086]
[0087] R f = 0.53 (PE / EtOAc = 9:1); 1 H NMR (600MHz, CDCl 3 )δ3.61(s,2H),2.37(s,3H),1.45(s,9H).
Embodiment 2
[0089] Synthesis of intermediate A1-2 with X=Y=CH, R 1 = Me as an example.
[0090]
[0091] Add 2-chloro-3-cyano-6-picoline (50.35 g, 0.330 mol), tert-butyl 2-acetylthioacetate (69.06 g, 0.363 mol) and DMF (330 mL) into a reaction flask (500 mL) ), cooled to 0–5°C, added NaOMe (21.39g, 0.396mol) in batches, and reacted at room temperature for 1h. The reaction solution was poured into water (3L), and a large amount of yellow solids precipitated out. Suction filtration, washing with water until neutral, and drying gave tert-butyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate ( Yellow solid 75.51g, yield 87%).
[0092]
[0093] R f = 0.36 (PE / Acetone = 17:3); ESI-MS: 265.0 [M+H] + ; 1 H NMR (600MHz, CDCl 3 )δ7.77(d, J=8.3Hz,
[0094] 1H), 7.13(d, J=8.3Hz, 1H), 5.79(s, 2H), 2.66(s, 3H), 1.59(s, 9H).
Embodiment 3
[0096] Synthesis of Carboxylic Acid A1 with X=Y=CH,R 1 = Me as an example.
[0097] Add 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylic acid tert-butyl ester (75.34g, 0.285mol), NaHCO 3 (47.89g, 0.570mol) and DCM (285mL), trifluoroacetic anhydride (71.83g, 0.342mol) was added dropwise under stirring at room temperature, and the reaction was continued for 0.5h at room temperature after the dropwise addition was completed. The reaction solution was added dropwise with water (200mL), stirred until no gas was produced, the DCM layer was separated, the aqueous phase was extracted with DCM (200mL×2), the organic phases were combined, washed with saturated NaCl solution (200mL×2), and washed with anhydrous NaCl 2 SO 4 Drying, concentration under reduced pressure, multiple recrystallizations from MeOH gave tert-butyl 3-trifluoroacetylamino-6-methylthieno[2,3-b]pyridine-2-carboxylate (light yellow solid 93.26g, yield 91%).
[0098]
[0099] R f = 0.57 (PE / EtOAc = 17:3); ESI-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com