Preparation method and application of drug sustained-release carrier hydrogel
A slow-release carrier and hydrogel technology, which is applied in the application fields of drug sustained-release carrier hydrogel preparation, loading and sustained release, can solve problems that have not been reported, and achieve a wide range of applications, improve utilization, and provide good drugs. The effect of sustained release properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A preparation method of a drug sustained-release hydrogel (CDGM) based on bisacrylamide β-cyclodextrin / methacrylylated gelatin is as follows:
[0043] (1) Bisacrylamide β-cyclodextrin β-CDA 2 Preparation of:
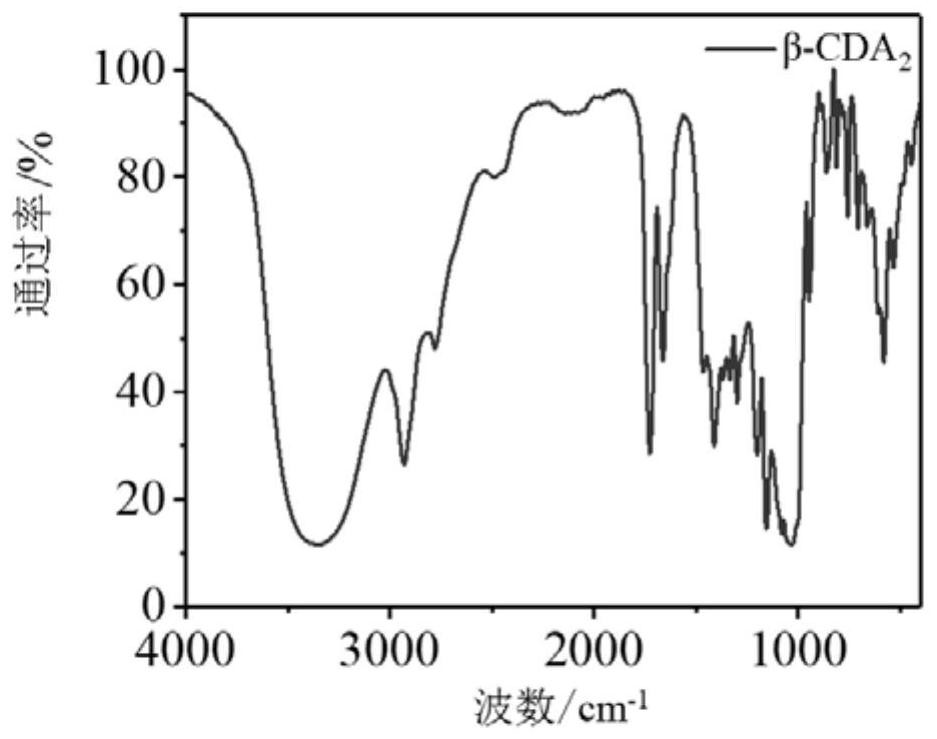
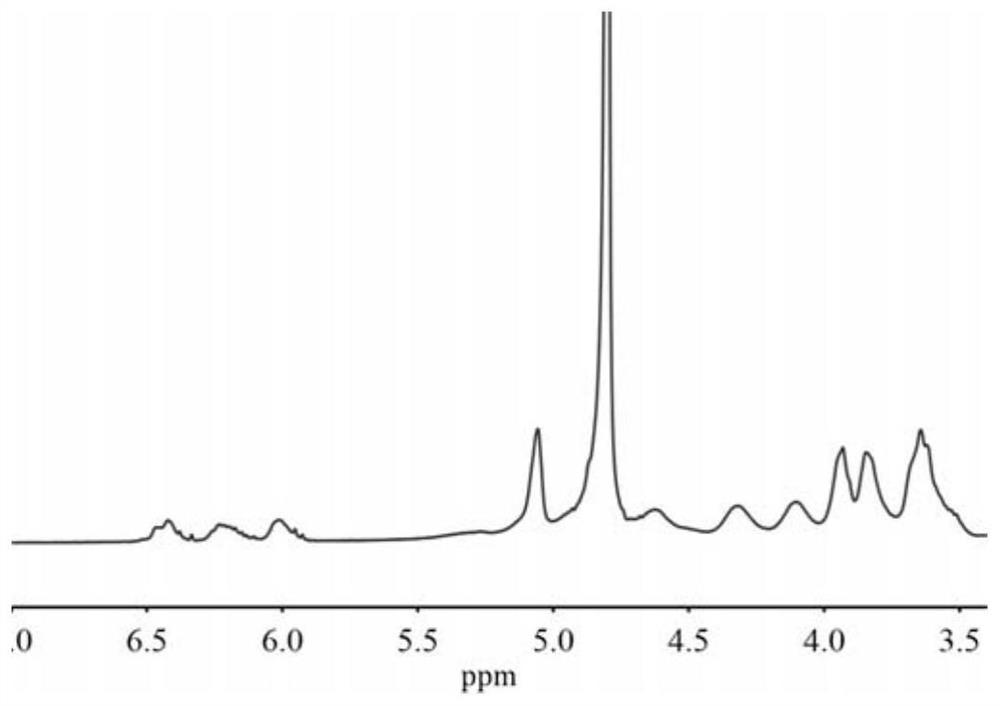
[0044] Dissolve 5g (4.4mmol) of β-cyclodextrin in N,N-dimethylformamide, pass through nitrogen, add triethylamine, and slowly add 2.85mL (35.2mmol) of acryloyl chloride dropwise to the above solution under ice-cooling (The dropping time is controlled to be 30 min). After stirring at room temperature for 1 hour, the triethylamine hydrochloride precipitate was removed by filtration, and the filtrate was precipitated with cold acetone, and the crude product was obtained by filtration. The crude product was dissolved in methanol and centrifuged to remove the cross-linked product part. The supernatant was concentrated under reduced pressure and then filtered with cold acetone. 2 ). Characterization of β-CDA by infrared spectroscopy 2 ,Such as figure 1 shown; throu...
Embodiment 2
[0055] A preparation method of CDGM drug carrier hydrogel is as follows:
[0056] (1) β-CDA 2 Preparation of:
[0057] With the step (1) of embodiment 1;
[0058] (2) Preparation of GelMA:
[0059] With the step (2) of embodiment 1;
[0060] (3) Preparation of CDGM drug sustained-release hydrogel:
[0061] (a) Prepare a GelMA solution with a monomer concentration of 0.15 g / mL in a three-necked flask, the solvent is PBS buffer, and stir to dissolve at 50 ° C;
[0062] (b) 6wt% β-CDA of solution is added in the there-necked flask 2 , 0.5wt% initiator Irgacure2959 of the solution, stirring and dissolving at 50°C;
[0063] (c) After stirring evenly, draw the liquid in the flask with a syringe, then slowly inject it into the pre-made glass mold, and then crosslink the glass mold under a 365nm ultraviolet lamp for 1 hour;
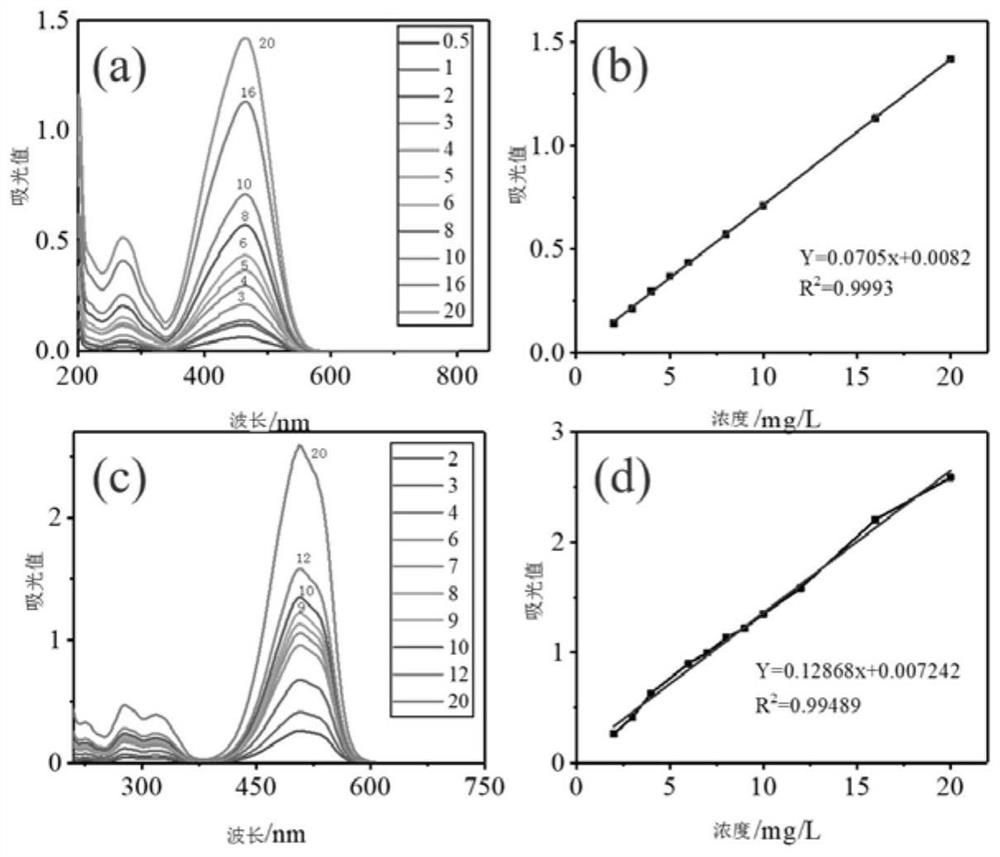
[0064] (d) Take out the hydrogel and soak it in deionized water for 24 hours to remove unreacted monomers, and wipe the surface of the hydrogel with filte...
Embodiment 3
[0068] A preparation method of CDGM drug carrier hydrogel is as follows:
[0069] (1) β-CDA 2 Preparation of:
[0070] With the step (1) of embodiment 1;
[0071] (2) Preparation of GelMA:
[0072] With the step (2) of embodiment 1;
[0073] (3) Preparation of CDGM drug sustained-release hydrogel:
[0074] (a) Prepare a GelMA solution with a monomer concentration of 0.15 g / mL in a three-necked flask, the solvent is PBS buffer, and stir to dissolve at 50 ° C;
[0075] (b) 9wt% β-CDA of solution is added in the there-necked flask 2 , 0.5wt% initiator Irgacure2959 of the solution, stirring and dissolving at 50°C;
[0076] (c) After stirring evenly, draw the liquid in the flask with a syringe, then slowly inject it into the pre-made glass mold, and then crosslink the glass mold under a 365nm ultraviolet lamp for 1 hour;
[0077] (d) Take out the hydrogel and soak it in deionized water for 24 hours to remove unreacted monomers, and wipe the surface of the hydrogel with filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com