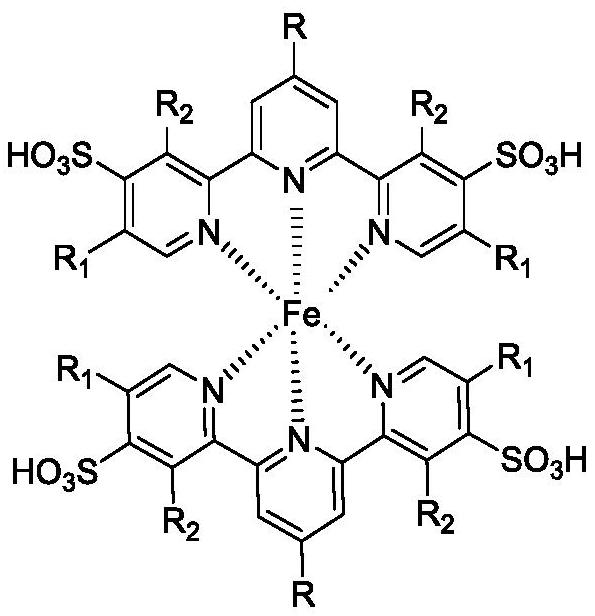
A kind of vanillin synthesis catalyst and preparation method thereof
A catalyst and a technology for vanillin, applied in the field of vanillin synthesis catalyst and its preparation and preparation, can solve the problems of low vanillin yield, reduced reaction time, over-oxidation and the like, achieve simple process, improve production efficiency and Yield, the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Catalyst preparation:
[0031] Pour pyridine and tetrahydrofuran solvents into a water-free and oxygen-free airtight three-neck flask, and slowly add butyllithium solution dropwise to the reaction system at -100°C. Quickly add trimethyltin chloride solution to the mixture, raise the temperature to 80°C for 6 hours, separate and purify to obtain the product 1. The added molar ratio of pyridine, trimethyltin chloride, and butyllithium is 1:1:1.
[0032] The product 1 was dissolved in toluene solution, 2-bromo-4sulfonic acid pyridine was added thereto, heated to 60°C and refluxed for 12 hours, and the product 2 was obtained by separation and purification. The added molar ratio of the 2-bromo-4sulfonic acid pyridine to the product 1 is 2:1. Product 2 is carried out proton nuclear magnetic resonance spectrum analysis, and the results are as follows: 1 H NMR (600MHz, CDCl 3 ): δ2.0(2H), 7.79(2H), 7.82(1H), 8.69(2H), 9.04(2H), 9.17(2H).
[0033] Mix product 2 and ferrous ...
Embodiment 2
[0035] Catalyst preparation:
[0036] Inject 4-picoline and tetrahydrofuran solvent into a water-free and oxygen-free airtight three-neck flask, slowly add butyllithium solution dropwise to the reaction system at -80°C, after the dropwise addition, raise to room temperature and continue the reaction for 3h , quickly added trimethyltin chloride solution to the reaction bottle, raised the temperature to 50°C for 8 hours, and separated and purified to obtain product 1. The molar ratio of 4-picoline, trimethyltin chloride and butyllithium is 1:1:2
[0037] The product 1 was dissolved in toluene solution, 2-bromo-3-methyl-4-sulfonic acid pyridine was added thereto, heated to 200° C. for reflux reaction for 12 hours, and the product 2 was obtained by separation and purification. The added molar ratio of the 2-bromo-3-methyl-4sulfonic acid pyridine to the product 1 is 5:1. Product 2 is carried out proton nuclear magnetic resonance spectrum analysis, and the results are as follows: ...
Embodiment 3
[0040] Catalyst preparation:
[0041]Inject 4-ethylpyridine and tetrahydrofuran solvent into a water-free and oxygen-free airtight three-neck flask, slowly add butyllithium solution dropwise to the reaction system at -50°C, after the dropwise addition, raise to room temperature and continue the reaction for 3h , quickly added trimethyltin chloride solution to the reaction flask, raised the temperature to 30°C for 12 hours, and separated and purified to obtain product 1. The added molar ratio of 4-ethylpyridine, trimethyltin chloride, and butyllithium is 1:2:2
[0042] The product 1 was dissolved in toluene solution, 2-bromo-4sulfonic acid-5-picoline was added thereto, heated to 80° C. and refluxed for 12 hours, and the product 2 was obtained by separation and purification. The added molar ratio of the 2-bromo-4sulfonic acid-5-picoline to the product 1 is 2.5:1. Product 2 is carried out proton nuclear magnetic resonance spectrum analysis, and the results are as follows: 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com