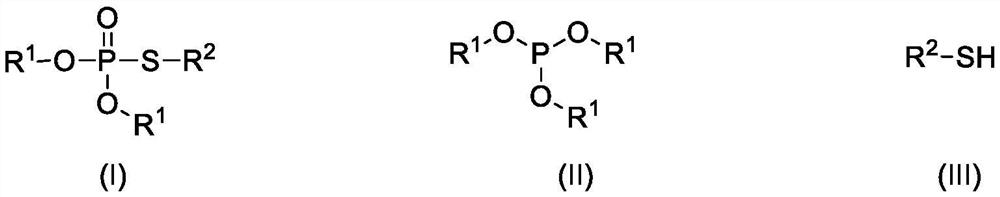
Direct electrochemical synthesis method of thiophosphate compound
A technology of phosphorothioate and synthesis method, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of high raw material price and long reaction time, and achieve the effect of reducing environmental cost and good universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
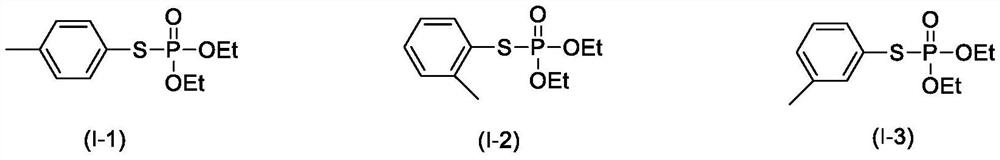
[0021] Example 1: O, O-diethyl-S - pair of phenylthio phosphate (preparation of formula (I-1))
[0022] Add 0.1mol / L bu in 30 ml of beakers 4 NBF 4 Ethyl acetonitrile solution (15 mL), triethylate (0.5 mmol) and toluene (0.9 mmol). 40 ° C, 1.0V disperse potential electrolysis, the reaction is completed after 4 hours. The solvent was evaporated under reduced pressure, and the column chromatography was separated, and the mixture of petroleum ether / ethyl acetate was 8: 1, the eluent containing the target compound was collected, and the solvent was evaporated. O-diethyl-S-p-p-phenylthio phosphate, separated yield is 68%.
Embodiment 2
[0023] Example 2: O, O-diethyl-S - pair of phenylthio phosphate (formula (I-1))
[0024] The reaction step is in Example 1, and the difference is 0.9 mmol, O, O-diethyl-S-S-Diethyl-S-S-S-Diethyl-S-S-Si-S-Diethyl-S- 55%.
Embodiment 3
[0025] Example 3: O, O-diethyl-S - preparation of toluene thiophosphate (formula (I-1))
[0026] The reaction step is the same as in Example 1, which is 0.75 mmol, O, O-diethyl-S-Di-sulphophosphate, is 51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com