Method for preparing 1, 4-dihydropyridine derivative containing azulene ring structure
A technology of dihydropyridine and its derivatives, which is applied in the field of medicine and chemical industry, can solve the problems that the catalyst cannot be recycled, the product purification process is complicated, and the catalytic activity is low, so as to reduce the reaction time and the amount of catalyst used, and improve economic benefits and environmental protection. Benefits, high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
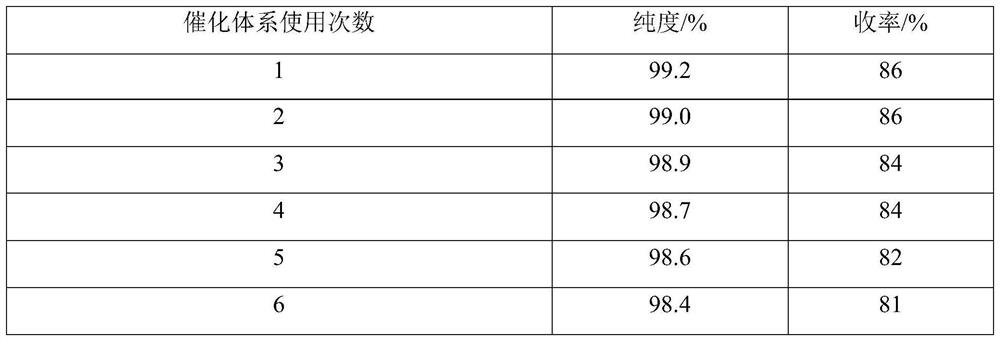
Embodiment 1
[0034] Add 1.0mmol 1-formyl azulene-3-carboxylic acid to a 50ml three-neck flask with a spherical condenser, a thermometer and a stirring bar containing 7ml of a mixed solvent (the volume ratio of tert-butanol-ethyl acetate is 9:1) Methyl ester, 1.6mmol methyl acetoacetate, 1.8mmol ammonium acetate, stirred at room temperature, mixed uniformly, then added 0.03mmol acidic ionic liquid, mixed uniformly. Heating in an oil bath, uniformly warming up to reflux (steam does not exceed the second ball of the spherical condenser), keeping the reflux reaction for 142min, TLC (silica gel thin plate chromatography, developing agent is V (benzene): V (ethyl acetate) = 93 : 7) monitoring, the raw material point disappears, and the reaction ends. Turn off the heating and stirring, the reaction liquid is naturally cooled to room temperature, a large amount of solids precipitate out, crush the solids, let stand for 36 hours, filter under reduced pressure, filter the residue through a mixed sol...
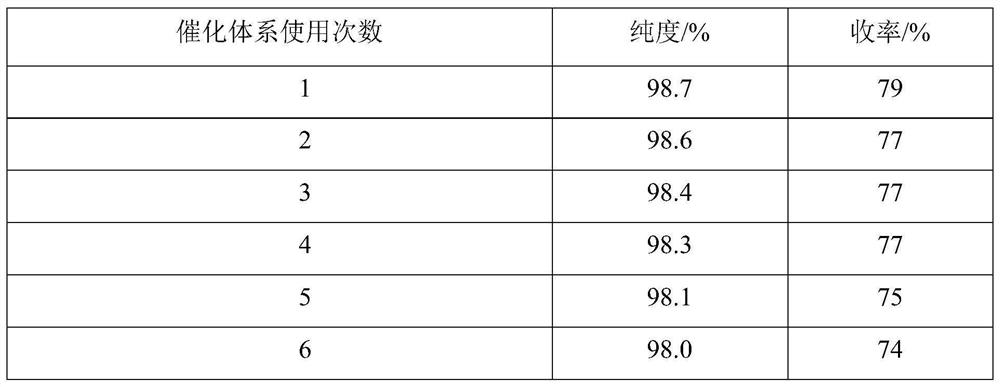
Embodiment 2
[0039] Add 1.0mmol 1-formyl azulene-3-carboxylic acid to a 50ml three-neck flask with a spherical condenser, a thermometer and a stirring bar containing 8ml of a mixed solvent (the volume ratio of tert-butanol-ethyl acetate is 9:1.2) Methyl ester, 1.7mmol ethyl acetoacetate, 2.0mmol ammonium acetate, stirred at room temperature, mixed evenly, then added 0.04mmol acidic ionic liquid, mixed evenly. Heat in an oil bath, evenly heat up to reflux (steam does not exceed the second ball of the spherical condenser), keep the reflux reaction for 158min, TLC (silica gel thin plate chromatography, developing solvent is V (benzene): V (ethyl acetate) = 92 : 8) monitoring, the raw material point disappears, and the reaction ends. Turn off the heating and stirring, the reaction liquid is naturally cooled to room temperature, a large amount of solids are precipitated, the solids are crushed, left to stand for 36 hours, vacuum filtered, and the filter residue is passed through a mixed solvent...
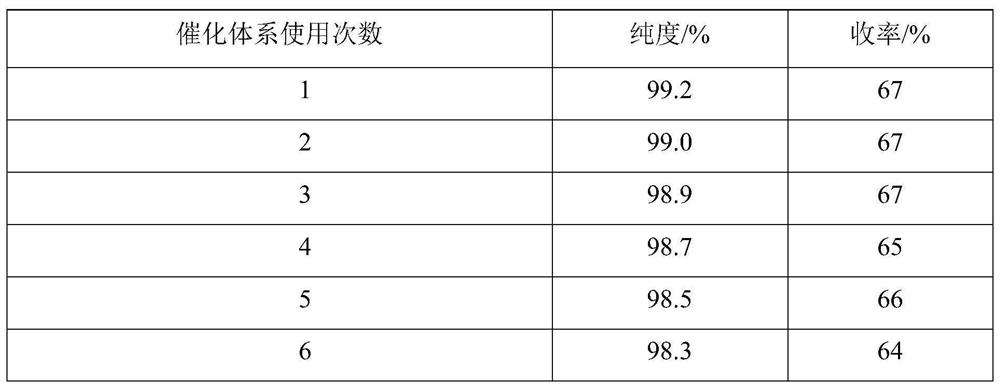
Embodiment 3
[0044] Add 1.0mmol 1-formyl azulene-3-carboxylic acid to a 50ml three-neck flask with a spherical condenser, a thermometer and a stirring bar containing 8ml of a mixed solvent (the volume ratio of tert-butanol-ethyl acetate is 9:1.3) Methyl ester, 1.8mmol isopropyl acetoacetate, 2.0mmol ammonium acetate, stirred at room temperature, mixed evenly, then added 0.04mmol acidic ionic liquid, mixed evenly. Heating in an oil bath, uniformly warming up to reflux (steam does not exceed the second ball of the spherical condenser), keeping the reflux reaction for 181min, TLC (silica gel thin plate chromatography, developing solvent is V (benzene): V (ethyl acetate) = 92 : 8) monitoring, the raw material point disappears, and the reaction ends. Turn off the heating and stirring, the reaction liquid is naturally cooled to room temperature, and a large amount of solids are precipitated, crushed solids, left to stand for 36 hours, and filtered under reduced pressure, and the filter residue i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com