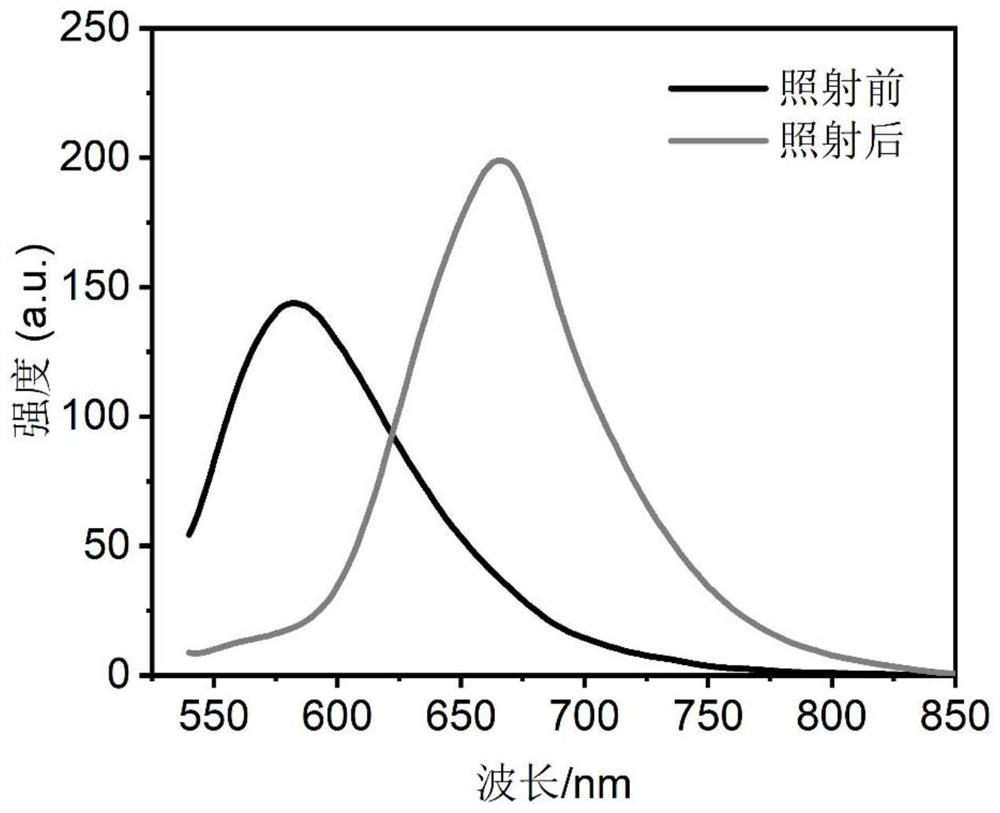
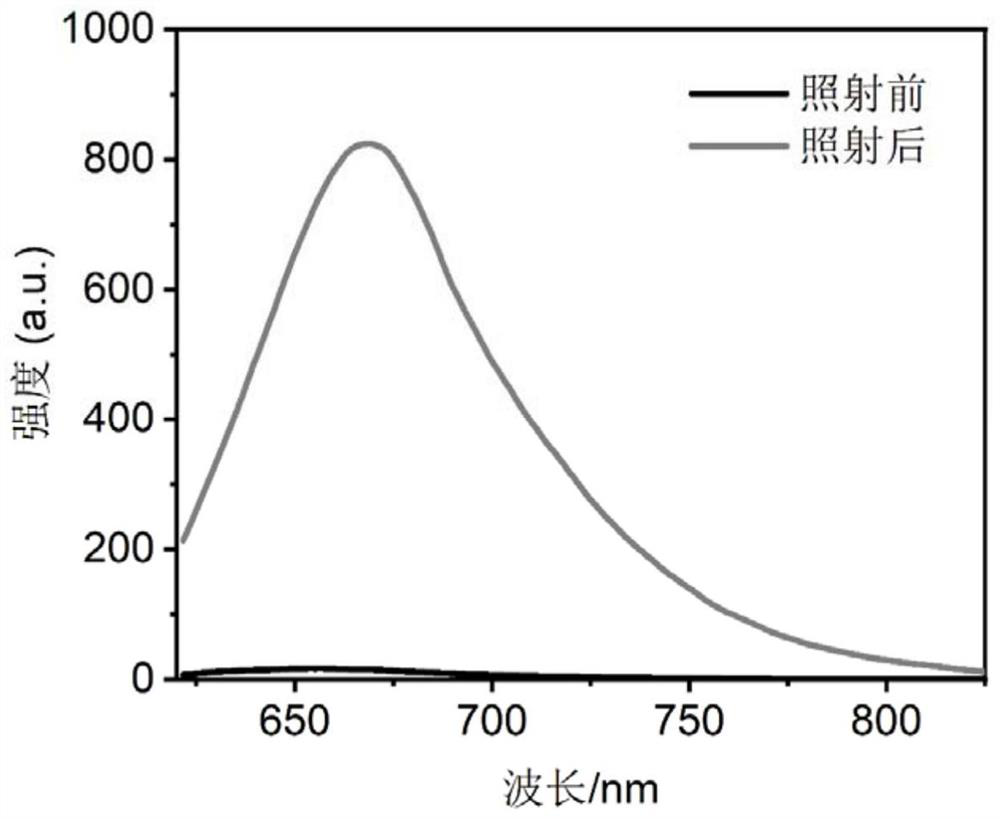
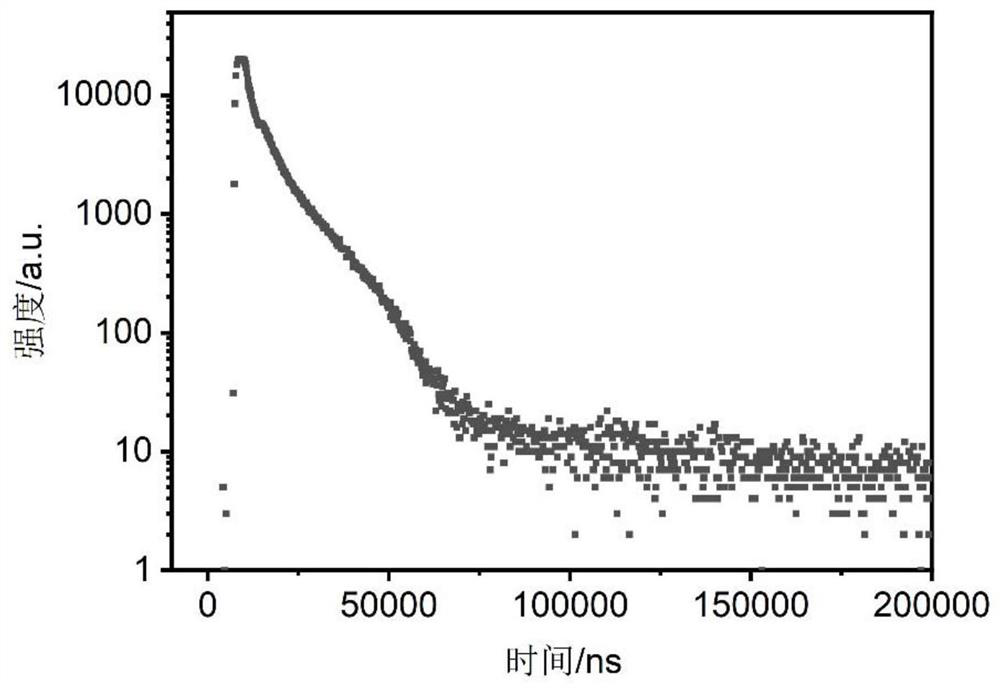
Spiropyran derivative, photochromic material and preparation method of photochromic material
A technology of photochromic materials and spiropyran derivatives, applied in color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of reduced detection accuracy and interference, and achieve low equipment investment and stable luminescent properties , The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] [Preparation method of photochromic material]
[0059] The present invention also provides a preparation method of a photochromic material as shown above, which includes the following steps:
[0060] (1), dissolving the host material in the first solvent to obtain the first mixture; dissolving the guest material in the second solvent to obtain the second mixture;
[0061] (2) Mix the first mixture and the second mixture, then remove the first solvent and the second solvent, and obtain the photochromic material after drying. Preferably, a vacuum distillation method can be used to remove the first solvent and the second solvent in the mixture system.
[0062] Optionally, the preparation method of the photochromic material may also be: dissolving the host material and the guest material in the same solvent at the same time, then removing the solvent, and drying to obtain the photochromic material. Similarly, vacuum distillation can also be used to remove the solvent in t...
Embodiment 1
[0066] This embodiment provides a photochromic material, which includes a host material and a guest material.
[0067] Wherein, the host material is polymethyl methacrylate, the structural formula is shown in the above formula (II-3), and n=10000.
[0068] The guest material is a chemical structure (abbreviated as compound 3) shown in formula (I), and the group R is
[0069] The synthetic method of compound 3 is: under reflux state, join following compound 1 (1.67g, 10mmol) in the ethanol solution (30mL) of following compound 2 (2.91g, 10mmol), reflux reaction 12 hours, then cooling After reaching room temperature, the solid was collected by filtration and finally recrystallized in ethanol to obtain compound 3 (1.3 g).
[0070]
[0071] The H NMR spectrum parameters of compound 3 are as follows: 1 H NMR (400MHz, CDCl 3 )δ8.06–7.98(m,2H),7.67(d,J=7.5Hz,1H),7.52–7.47(m,2H),7.35–7.32(m,1H),7.23–7.18(m,1H) ,6.93(d,J=10.4Hz,1H),6.88(s,1H),6.77(d,J=8.4Hz,1H),5.92(d,J=10.4Hz...
Embodiment 2
[0078] This embodiment provides a photochromic material, which includes a host material and a guest material.
[0079] Wherein, the host material is polyvinylpyrrolidone, the structural formula is shown in the above formula (II-6), and n=60000.
[0080] The guest material is a chemical structure (abbreviated as compound 6) shown in formula (I), and the group R is
[0081] The synthetic method of compound 6 is: under reflux state, join following compound 3 (1.67g, 10mmol) in the ethanol solution (30mL) of following compound 4 (3.05g, 10mmol), reflux reaction 12 hours, then cooling After reaching room temperature, the reaction solution was concentrated and purified by column to obtain compound 6 (0.81 g).
[0082]
[0083] The proton nuclear magnetic resonance spectrum parameters of compound 6 are as follows: 1 H NMR (400MHz, CDCl 3 )δ8.08–8.00(m,2H),7.56(d,J=7.3Hz,1H),7.44–7.33(m,2H),7.21(s,1H),7.16(td,J=7.3,1.0Hz ,1H),6.95(t,J=5.2Hz,2H),6.78(d,J=8.8Hz,1H),5.90(d,J=10....
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com