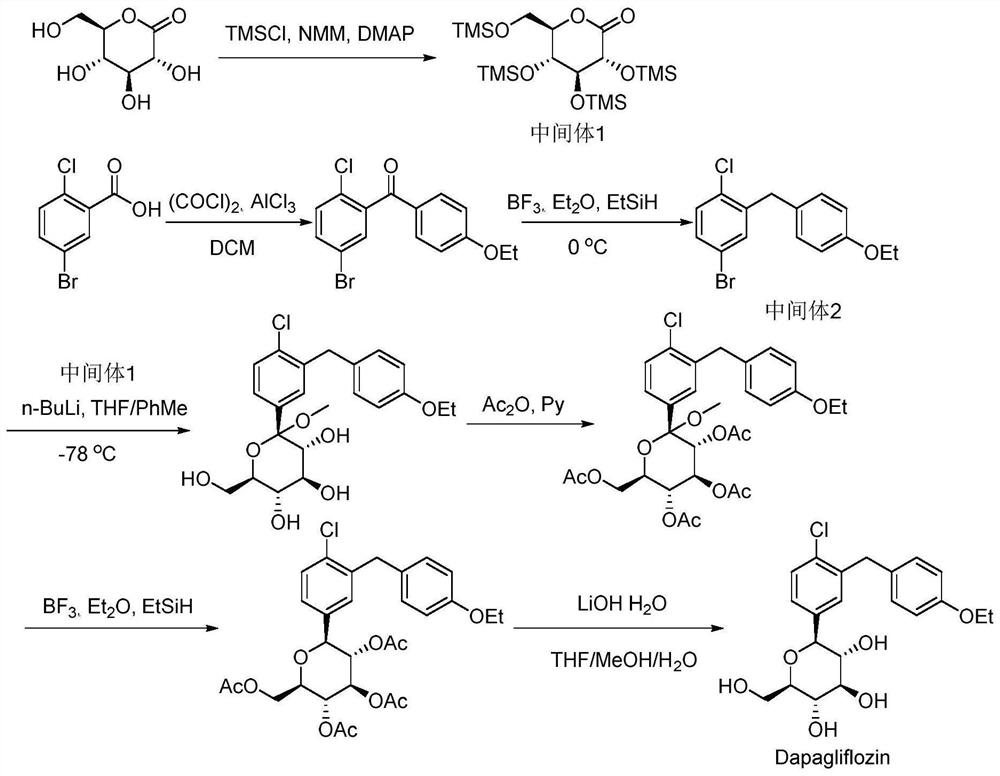
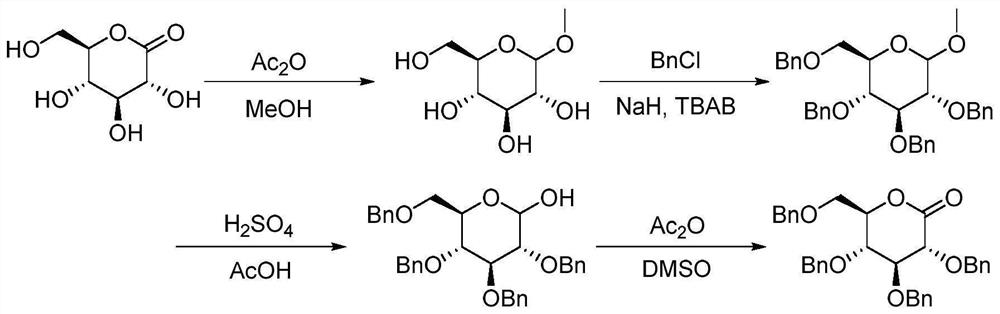
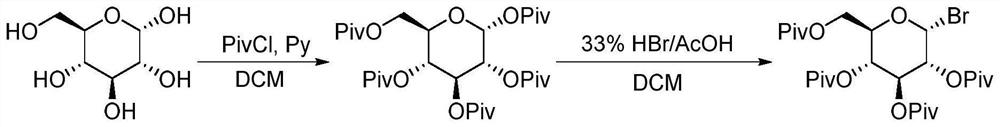
A method for continuously synthesizing benzyl-substituted gluconolactone using a microchannel reaction device
A technology of gluconolactone and microchannel reaction, which is applied in the direction of organic chemistry, can solve the problems of difficult purification of reaction products, complex reaction conditions, and long reaction time, and achieve simple and convenient post-processing, simple synthesis process, and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) In the microchannel reaction device, the tetrahydrofuran solution (50mL) of methyl-α-D-mannopyranoside (0.025mol) and the tetrahydrofuran solution (50mL) of benzyl chloride (0.125mol) are respectively pumped from pump A1, Pump B2, pumping into the first microstructure mixer 3, the flow rate of pump A1 is 0.25mL / min, and the flow rate of pump B2 is 1.25mL / min. Enter the first microreactor 4 after fully mixing, the volume of the first microreactor 4 is 25mL, and the reaction residence time is 20min. The reaction temperature was 55°C, and the yield was 93.2%.
[0045] (2) Along with the reaction liquid that step (1) obtains flows into the second microstructure mixer, the tetrahydrofuran solution (25mL) of hydrochloric acid (0.025mol) is pumped into the second Microstructure mixer 6, pump C5 flow rate is 0.39mL / min; After fully mixing, pump into the second microreactor 7 in the second section of continuous microchannel reaction device to react, the second microreactor ...
Embodiment 2
[0053] The operation is the same as in Example 1, the only difference is:
[0054] (1) In step (1), the flow rate of pump A 1 is 0.21 mL / min, and the flow rate of pump B 2 is 1.11 mL / min. The volume of the first microreactor 4 is 20mL, and the reaction residence time is 18min. The reaction temperature was 50°C, and the yield was 86.2%.
[0055] (2) The flow rate of the pump C5 is 0.31 mL / min, the volume of the second microreactor 7 is 20 mL, the residence time is 12 min, the heating temperature is 0° C., and the yield is 84.9%.
[0056](3) The flow velocity of the pump D8 is 0.675mL / min, the flow velocity of the pump E9 is 0.04mL / min, the volume of the third microreactor 11 is 20mL, the reaction temperature is 50°C, the residence time is 9min, and the productive rate is 81.8%. , with a purity of 97.5%.
Embodiment 3
[0058] The operation is the same as in Example 1, the only difference is:
[0059] (1) In step (1), the flow rate of pump A1 is 0.29mL / min, and the flow rate of pump B2 is 1.53mL / min. The volume of the first microreactor 4 is 30mL, and the reaction residence time is 22min. The reaction temperature was 75°C, and the yield was 87.5%.
[0060] (2) The flow rate of the pump C5 is 0.42mL / min, the volume of the second microreactor 7 is 30mL, the residence time is 16min, the heating temperature is 25° C., and the yield is 85.8%.
[0061] (3) The flow velocity of pump D8 is 0.70mL / min, the flow velocity of pump E9 is 0.105mL / min, the volume of the third microreactor 11 is 30mL, reaction temperature is 75 ℃, residence time is 14min, productive rate is 82.4% , with a purity of 96.8%.
[0062] This application is based on the difficulty of synthesizing the intermediate benzylgluconolactone through the above-mentioned traditional route. Through the study of reaction kinetics and the in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com