Method for continuously preparing Olaparib intermediate by microchannel modular reaction device
A technology of microchannel reaction and microchannel module, which is applied in the field of preparation of antineoplastic drug olaparib intermediates, can solve the problems of difficult purification, low yield, and many by-products, and achieve low toxicity and pollution, high profit, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
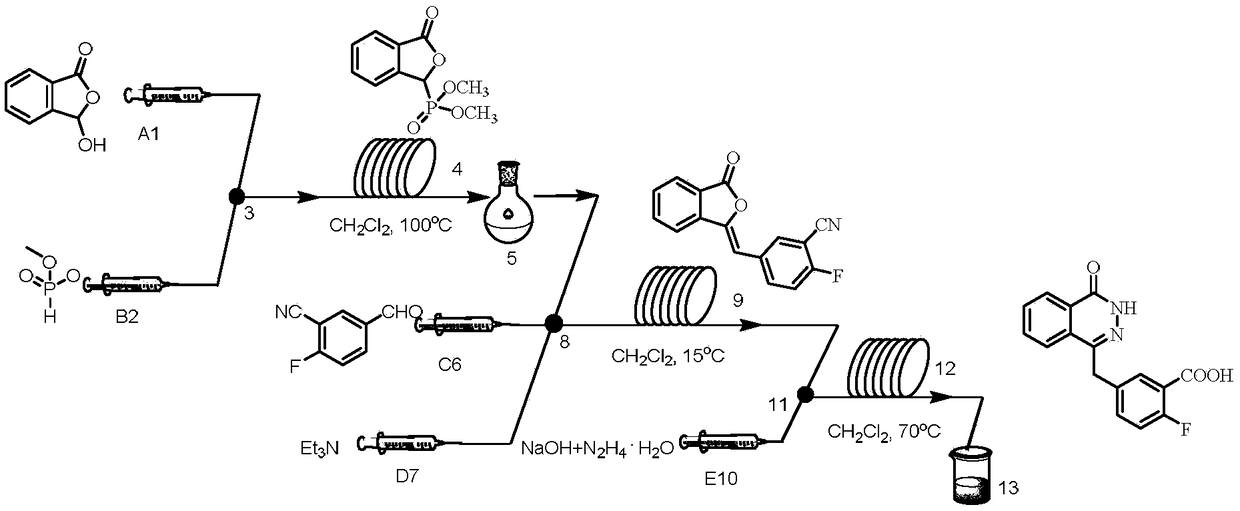
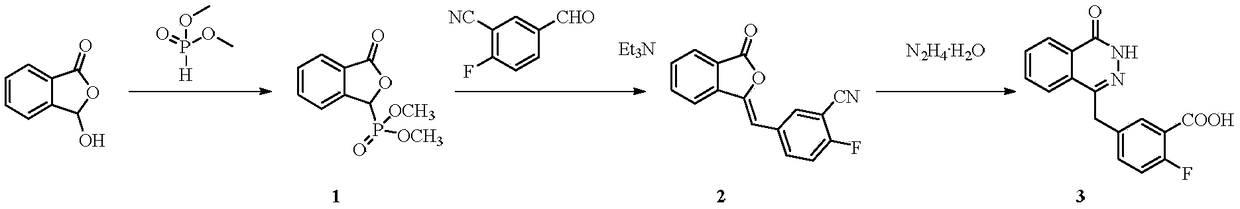
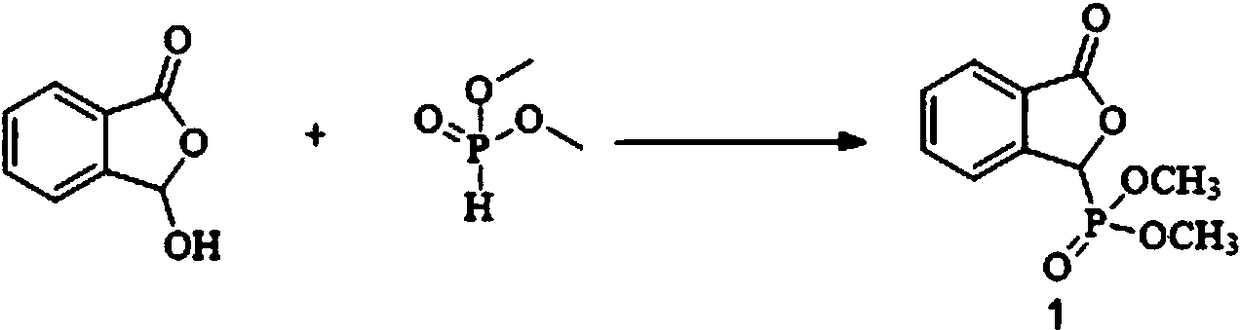
[0037] (1) In a microchannel reaction device, a dichloromethane solution (50 mL) of 3-hydroxyisobenzofuran-1 (3H)-one (0.033 mol) and a dichloromethane solution of dimethyl phosphite (0.091 mol) were mixed Methane solution (50 mL) was pumped into the first mixing valve 3 from pump A1 and pump B2 respectively, the flow rate of pump A1 was 0.18 mL / min, and the flow rate of pump B2 was 0.026 mL / min. Enter the first microreactor 4 after fully mixing, the volume of the first microreactor 4 is 40mL, and the reaction residence time is 25min. The reaction temperature is 100°C, and the reaction liquid is collected in the first separation device 5, which is a container for holding water and dichloromethane, and the obtained (3-oxo-1,3- Dihydroisobenzofuran-1-yl) dimethyl dihydroisobenzofuran-1-yl) phosphate (compound 1) effluent, yield 85%.
[0038] (2) As the reaction solution obtained in step (1) flows into the second mixing valve, the flow rate is 1.24mL / min, and a dichloromethane s...
Embodiment 2
[0049] The operation is the same as in Example 1, the only difference is:
[0050] In step (1), the reaction temperature in the first microreactor is 90°C, and finally (3-oxo-1,3-dihydroisobenzofuran-1-yl) dimethyl phosphate (compound 1) is obtained The effluent, the yield was 76%.
Embodiment 3
[0052] The operation is the same as in Example 1, the only difference is:
[0053] In step (1), the reaction temperature in the first microreactor is 120°C, and finally (3-oxo-1,3-dihydroisobenzofuran-1-yl) dimethyl phosphate (compound 1) is obtained The effluent, the yield was 72%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com