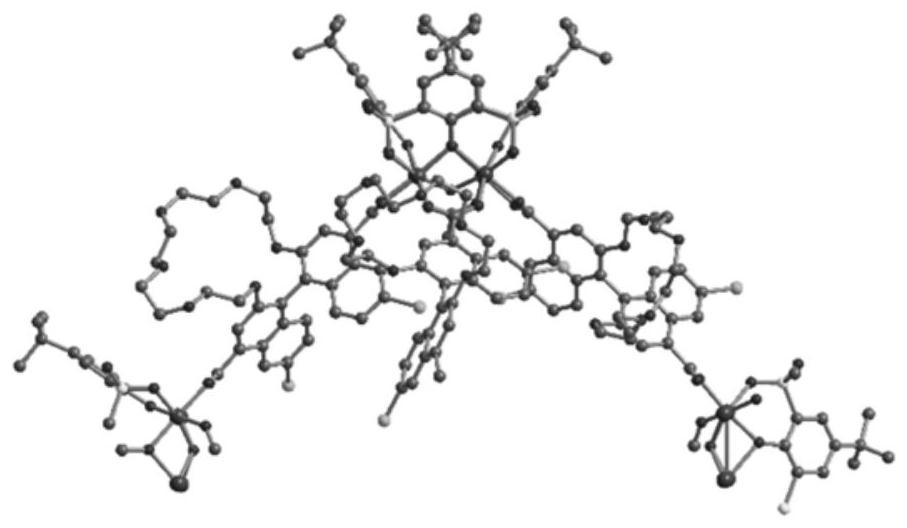
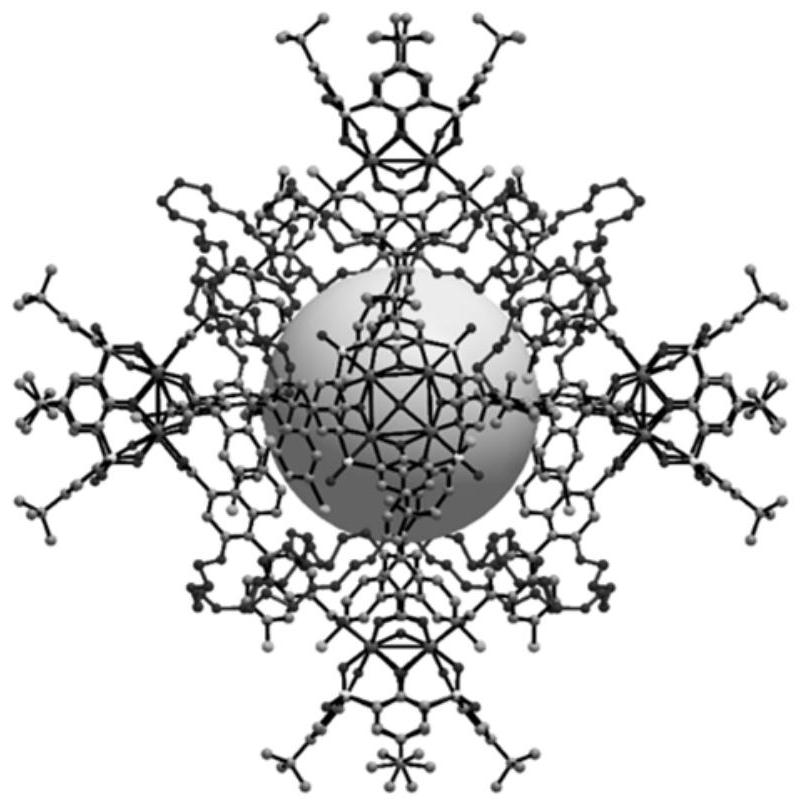
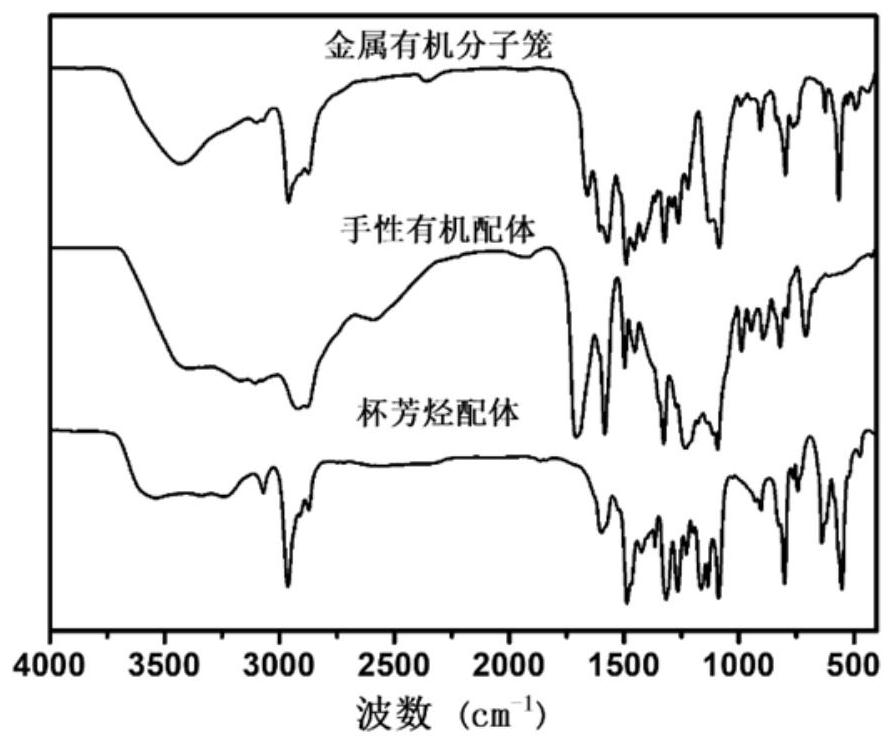
Mixed matrix membrane taking chiral metal organic molecular cage as filler as well as preparation and application of the mixed matrix membrane
A technology of metal-organic molecules and mixed matrix membranes, which is applied in membrane technology, semi-permeable membrane separation, chemical instruments and methods, etc., can solve problems such as uneven mixing, poor mechanical properties, and poor film-forming properties, and overcome uneven mixing , high stability, good flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation method of the mixed matrix membrane with the chiral metal-organic molecular cage as the filler comprises the following steps:
[0054] 1. Preparation of chiral binaphthalene dicarboxylic acid ligand functionalized with crown ether:
[0055]
[0056] Add 5.0 g of starting material compound S1 to a 250 mL dry two-necked round bottom flask, evacuate nitrogen three times, and then add 60.0 mL of dry CH 2 Cl 2 , and the temperature of the solution was lowered to -78° C., and 2.85 mL of boron tribromide in dichloromethane (20.0 mL) was added dropwise. Slowly return to room temperature and stir overnight. After the reaction was completed, the reaction solution was poured into an ice-water bath to quench the reaction. Dichloromethane was removed under reduced pressure, ethyl acetate was added to extract and the organic phase was collected, dried and spin-dried. The crude product was separated by a short silica gel column to obtain 4.5g of white compound S...
Embodiment 2
[0073] The preparation method of the mixed matrix membrane with the chiral metal-organic molecular cage as the filler comprises the following steps:
[0074] 1) Using 6,6'-dichloro-2,2'-diethoxy-[1,1'-binaphthalene]-4,4'-dicarboxylic acid as the starting material, successively deprotect the hydroxyl group, Esterification of carboxyl groups, functionalization of crown ethers and hydrolysis of ester groups to obtain chiral binaphthyl dicarboxylic acid ligands functionalized with crown ethers. The consumption of boron tribromide is 3 times of equivalents; the consumption of pentylene glycol di-p-toluenesulfonate is 5 times of equivalents; the consumption of lithium hydroxide is 5 times of equivalents. Specifically include:
[0075] 1-1) Compound S1, boron tribromide and dichloromethane were mixed, and the ethyl group of compound S1 was removed by reaction to obtain hydroxyl-containing compound S2; the reaction conditions were: stirring overnight at room temperature.
[0076] 1-...
Embodiment 3
[0084] The preparation method of the mixed matrix membrane with the chiral metal-organic molecular cage as the filler comprises the following steps:
[0085] 1) Using 6,6'-dichloro-2,2'-diethoxy-[1,1'-binaphthalene]-4,4'-dicarboxylic acid as the starting material, successively deprotect the hydroxyl group, Esterification of carboxyl groups, functionalization of crown ethers and hydrolysis of ester groups to obtain chiral binaphthyl dicarboxylic acid ligands functionalized with crown ethers. The consumption of boron tribromide is 6 times of equivalents; the consumption of pentylene glycol di-p-toluenesulfonate is 2 times of equivalents; the consumption of lithium hydroxide is 8 times of equivalents. Specifically include:
[0086] 1-1) Compound S1, boron tribromide and dichloromethane were mixed, and the ethyl group of compound S1 was removed by reaction to obtain hydroxyl-containing compound S2; the reaction conditions were: stirring overnight at room temperature.
[0087] 1-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com