Multifunctional cross-linking agent as well as preparation method and application thereof
A cross-linking agent and multi-functional technology, applied in organic chemistry methods, chemical process analysis/design, organic chemistry, etc., can solve the problem of few reactive groups and inability to capture the dynamic spatial conformation or interaction of transmembrane proteins in situ Information and other issues, to achieve efficient and gentle release, improve identification accuracy, and achieve the effect of chemical cross-linking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
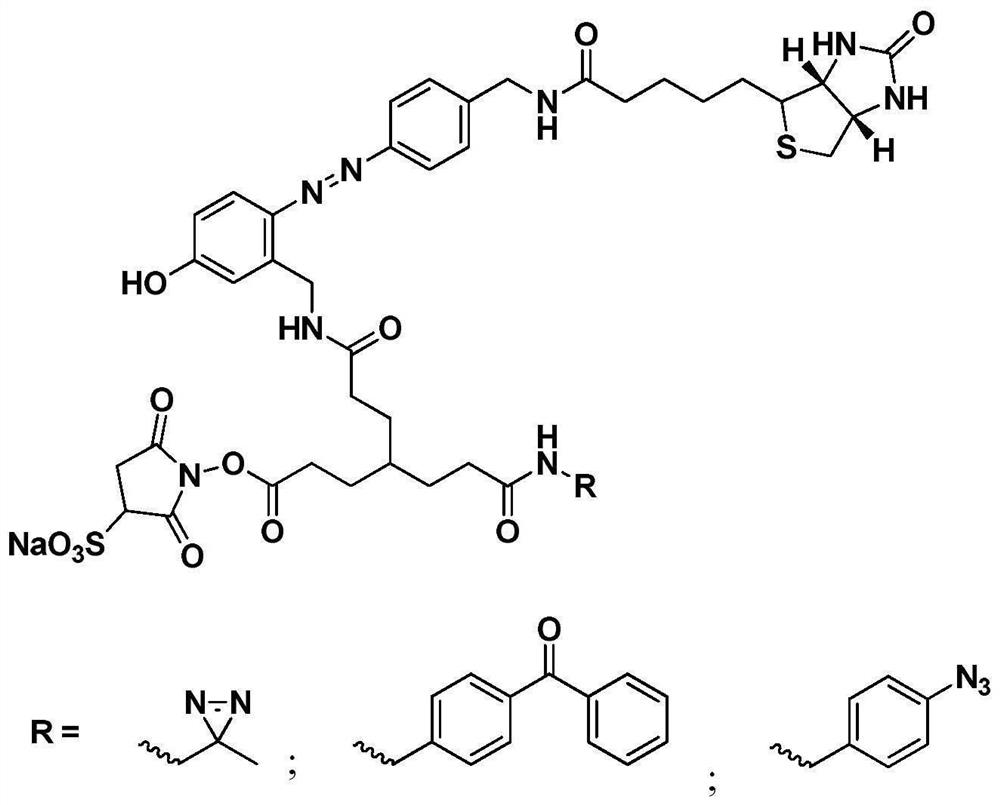
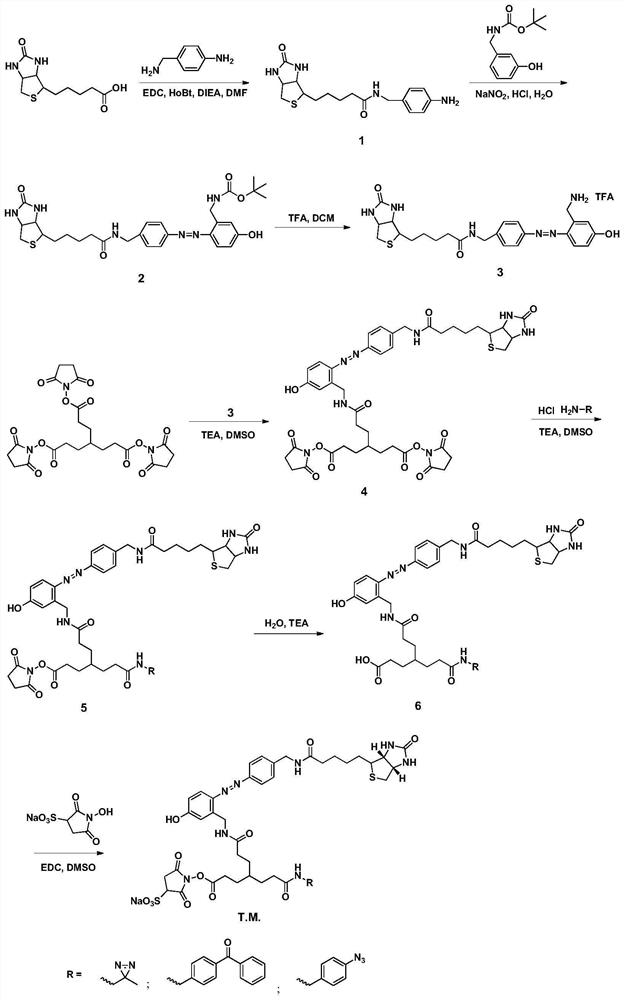
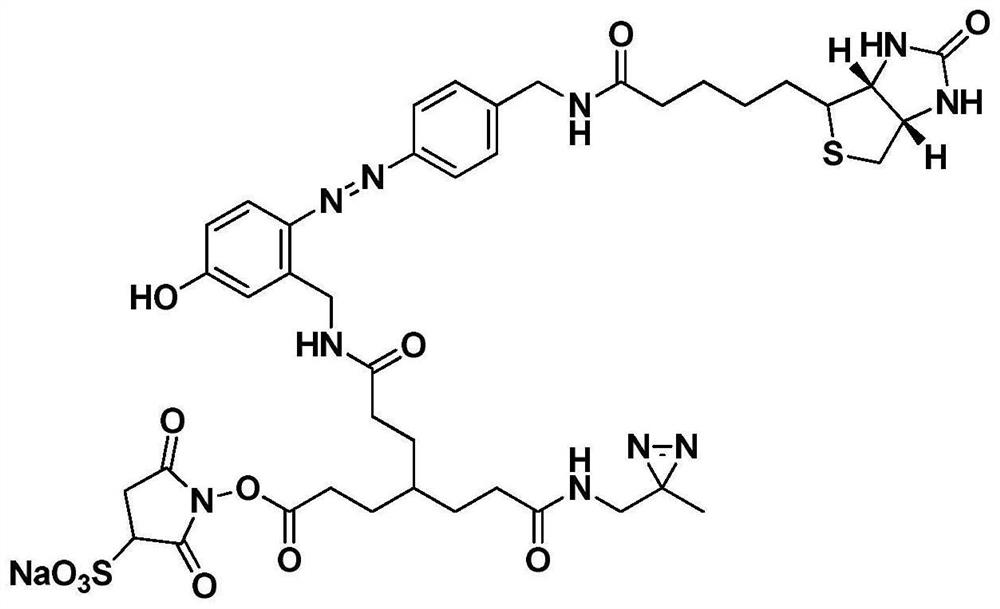
[0042] This example discloses a preparation method of a cross-linking agent in which diaziridine is a photoactive group, including seven reaction steps, and the preparation method is as follows:
[0043] The first step, the preparation of biotin aniline (compound 1). Dissolve biotin (1.22g, 5 mmol), 4-aminomethylaniline (670mg, 5.5mmol), EDCI (1.23g, 6.5mmol), HoBt (988mg, 6.5mmol) in 25ml DMF, add DIEA (2ml , 20 mmol), reacted at 25°C for 24h. After the reaction was completed, the solvent was spin-dried under reduced pressure at 45° C., and excess raw materials were removed by column chromatography to obtain 1.72 g of a white solid with a yield of 98.8%. 1 H NMR (400MHz, DMSO-d6,ppm)δ5.83(s,2H),4.76(s,1H),2.80(s,8H),2.72(d,J=4.8Hz,2H),2.65-2.60( m,2H); HR-MS (C15H16N6O9): theoretical value: 348.1620 measured value [M+H]: 349.1589.
[0044] The second step is the preparation of biotinylated azodiphenyl Boc aminomethylphenol (compound 2). Add compound 2 (1.04g, 3mmol), conc...
Embodiment 2
[0053] This embodiment discloses a preparation method of a crosslinking agent in which benzophenone is a photoactive group, including seven reaction steps, and the preparation method is as follows:
[0054] Wherein, the first, second, third and fourth steps are the same as in embodiment 1.
[0055] The fifth step is the preparation of biotin azodiphenyl monosuccinimide benzophenone ester (compound 5-b). Dissolve compound 4 (204.7mg, 0.23mmol) and 66ul TEA in 20ml DMSO, slowly add benzophenone amino hydrochloride (56.8mg, 0.23mmol) DMSO solution dropwise, react for 0.5h, and undergo semi-preparative liquid phase separation Purification (40% acetonitrile isocratic, effluent was collected for 24-27 min) and vacuum freeze-drying at 25°C to obtain 156 mg of red solid compound 5-b with a yield of 68.9%. 1H NMR (400MHz, DMSO-d6, ppm) δ4.76(s,1H),3.36(t,J=6.4Hz,1H),3.10(s,2H),2.92(s,2H),2.86(m, 4H),2.83(s,1H),2.8(s,8H),2.65-2.60(m,2H),2.05(t,J=6.4Hz,2H), 1.57(m,2H),1.25(m,2H ); 13...
Embodiment 3
[0060] This example discloses a preparation method of a crosslinking agent in which phenyl azide is a photoactive group, including seven reaction steps, and the preparation method is as follows:
[0061]
[0062] Wherein, the first, second, third and fourth steps are the same as in embodiment 1.
[0063] The fifth step, the preparation of biotin azodiphenyl monosuccinimide ester phenyl azide (compound 5-c) Dissolve compound 4 (204.7mg, 0.23mmol), 66ul TEA in 20ml DMSO, slowly Add benzophenone amino hydrochloride (42.3mg, 0.23mmol) DMSO solution dropwise, react for 0.5h, undergo semi-preparative liquid phase separation and purification (30% acetonitrile isocratic, collect effluent for 24-27min) and vacuum freeze-drying, The freeze-drying temperature was 25°C, and 125 mg of red solid compound 5-c was obtained with a yield of 59%. 1 H NMR(400MHz,DMSO-d6,ppm)δ4.76(s,1H),2.92(s,2H),2.83(s,1H),2.80(s,8H),2.79(d,J=4.8Hz, 2H), 2.72(d, J=4.8Hz, 2H), 2.65-2.60(m, 2H); 13 C NMR (40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com