Anti-human IL-33 monoclonal antibody and application thereof
A monoclonal antibody, IL-33 technology, applied in applications, antibodies, anti-animal/human immunoglobulins, etc., can solve the high adverse reaction rate, unclear clinical application prospects, and anti-IL-33 antibodies need further research And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Preparation of anti-human IL-33 antibody mouse hybridoma cells
[0083] Human interleukin 33 (Ser112-Thr270) antigen was purchased from Novoprotein Technology Co., Ltd. (Novoprotein, product number C091), and 8-week-old Balb / c mice were selected as immunized animals, and blood was collected before immunization as a negative control. Human IL33 protein was emulsified in Freund's adjuvant and then subcutaneously injected for immunization three times with an interval of 2 weeks each time. Blood was collected from the tail vein one week after the third immunization to measure the antibody titer of the serum and select mice with high titer Sprint immunizations were performed 3 days prior to fusion.
[0084] Spleen cells and lymphocytes were aseptically separated from the spleen and lymph nodes of the immunized mice, fused with SP20 myeloma cells in good condition by electrofusion and PEG, and positive hybridomas were screened within 8 to 10 days. The hybridoma ce...
Embodiment 2
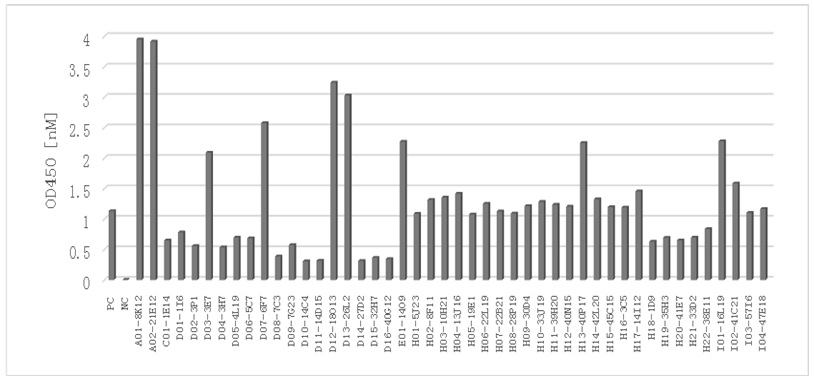
[0085] Example 2: The binding activity of anti-human IL33 monoclonal antibody secreted by hybridoma cell lines to human IL33
[0086] Take the supernatant of hybridoma cells 8-10 days after fusion, and use ELISA method to screen positive hybridoma mother clones that can secrete anti-human IL33 antibody.
[0087] The specific method is as follows: 0.5ug / ml human IL-33 protein, 30μl / well, coated 384-well plate (greiner bio-one 781097) and incubated overnight at 4°C; the next day, the plate was washed with PBST, and 60μl of IL-33 was added to the sample well Contain 1% BSA in PBST, block at 37°C for 2 hours; then wash the plate with PBST, and dry the water in the wells. Add 20 µl of the sample to be tested into the well plate and incubate at room temperature for 1 h. Then, after washing the plate with PBST, 30ul of 1:30000 diluted secondary antibody goat anti-mouse IgG coupled to horseradish peroxidase (Jackson, cat#: 115-035-164) was added to each well, and incubated at room te...
Embodiment 3
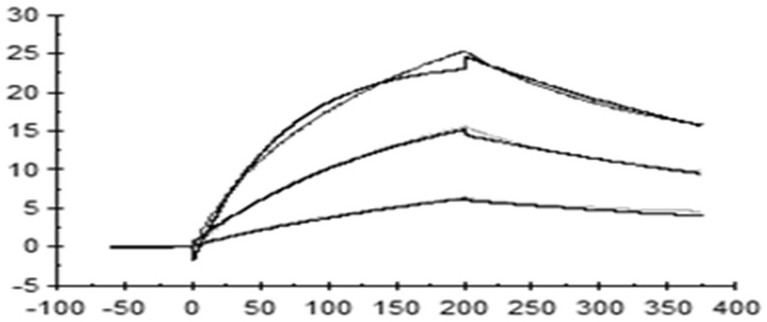
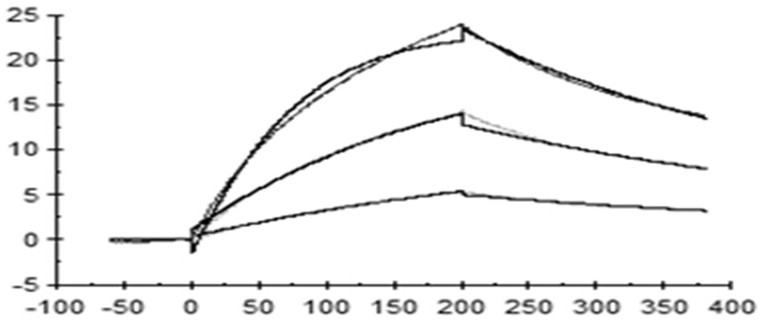
[0089] Example 3: Murine anti-human IL33 antibody blocks the binding of IL-33 to human ST2
[0090] Transfer the positive clones that bind to human IL33 protein from the 384-well plate to the 96-well plate, and use the ELISA method for blocking experiments. The specific method is as follows: coat the 96-well plate with hST2-hFc protein (Acro, #IL1-H5250), 1ug / ml, 50ul per well, incubated overnight at 4°C; the next day, wash the plate 3 times with PBST, add 200µl of PBST containing 1% BSA to the sample wells, block at 37°C for 2h; then wash the plate with PBST, and spin dry the wells Medium moisture. Add 50ul of pre-incubated samples (biotin hIL-33 protein and hybridoma clone supernatant) to the well plate and incubate at room temperature for 1 h. Then, after washing the plate with PBST, 50ul of 1:5000 diluted secondary antibody StrepTactin-HRP Conjugate (Bio-rad, #1610380) was added to each well, and incubated at room temperature for 1h. Wash the plate with PBST, add 50µl TM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com