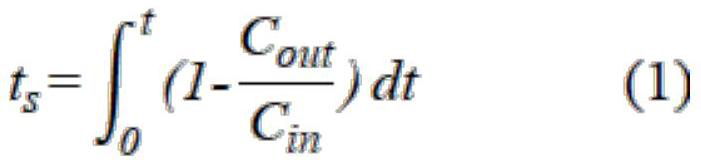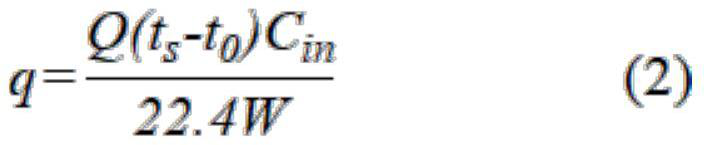Bimetal MOFs material for CO2 capture and preparation method and application thereof
A bimetal and metal technology, applied in the field of bimetal MOFs materials and preparation, can solve the problems of large steric hindrance effect, unfavorable carbon dioxide adsorption, decrease in specific surface and pore volume, etc., and achieves low equipment corrosion and simple preparation method. , the effect of easy regeneration process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
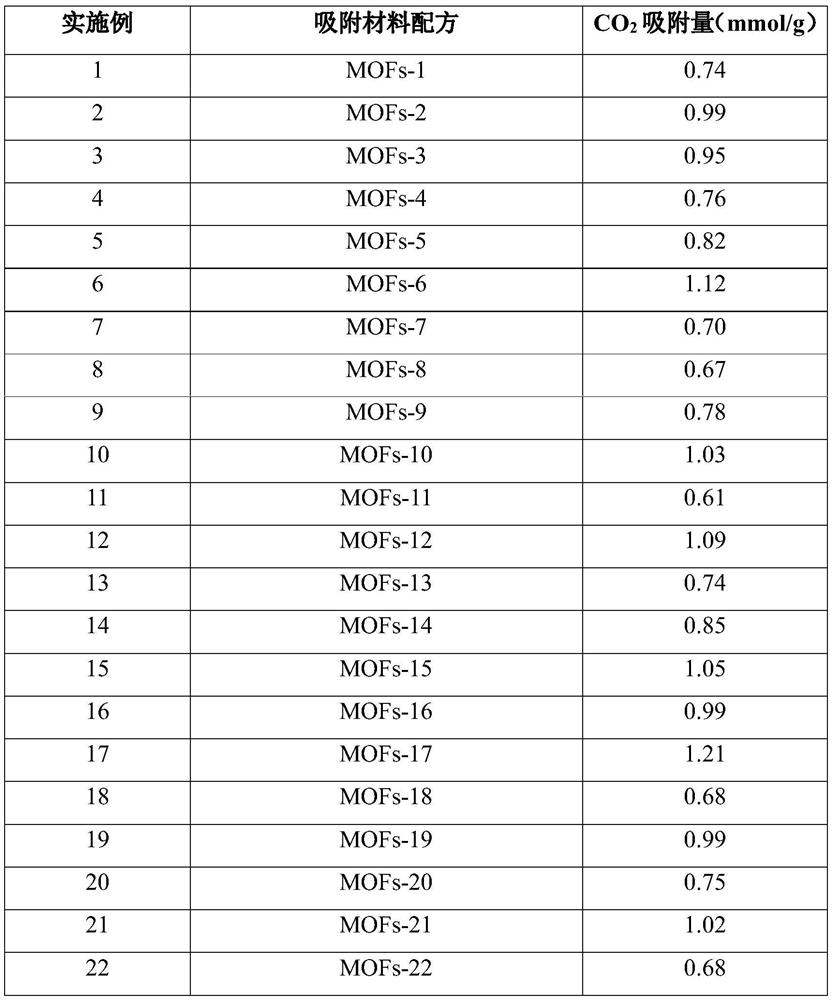
[0037] 9.50 mmol Timethylene, 12.825 mmol nitrate hydrate and 1.425 mmol of nickel nickel hydrate were dissolved in a mixed solution of 32 mL of N, N-dimethylformamide / ethanol, respectively. The above two solutions were moved into the glass bottle, heated at 60 ° C for 36 h. After the reaction was completed, the centrifugation was washed, dried in vacuo to give a bimetallic MOFS material MOFS-1.
[0038] The above-described adsorbed material was sieved, and 0.5 g of the adsorbent was loaded into the quartz tube, and gas adsorption was performed on a fixed bed. First, the adsorbent activates 3 h at 120 ° C, argon protection, and air speed is 4000 ml h. -1 . Subsequent Co at 25 ° C 2 Gas adsorption (mixed gas CO 2 : N 2 = 15: 85), air speed is 4000ml h -1 The gas adsorbers detect the exhaust gas online. CO 2The amount of adsorption is shown in Table 1.
Embodiment 2
[0040] 9.50 mmol 均 均 三 三 水, 11.40 mmol of nitrate hydrate and 2.85 mmol of nitrate hydrate were dissolved in a mixed solution of 32 mL of N, N-dimethylformamide / ethanol, respectively. The above two solutions were moved into the glass bottle, and 24 h was heated at 80 ° C. After the reaction, centrifugation was washed, dried in vacuo to give a bimetallic MOFS material MOFS-2.
[0041] The above-described adsorbed material was sieved, and 0.5 g of the adsorbent was loaded into the quartz tube, and gas adsorption was performed on a fixed bed. First, the adsorbent activates 2.5 h at 150 ° C, argon protection, and air speed of 3500 ml h. -1 . Subsequent CO at 35 ° C 2 Gas adsorption (mixed gas CO 2 : N 2 = 15: 85), air speed is 3500ml h -1 The gas adsorbers detect the exhaust gas online. CO 2 The amount of adsorption is shown in Table 1.
Embodiment 3
[0043] 9.50 mmol 均 均 三 三, 水 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶 溶. The above two solutions were moved into a glass bottle and heated at 120 ° C for 18 h. After completion of the reaction, the centrifugation was washed, and dried in vacuo to obtain a bimetallic MOFS material MOFS-3.
[0044] The above-described adsorbed material was sieved, and 0.5 g of the adsorbent was loaded into the quartz tube, and gas adsorption was performed on a fixed bed. First, the adsorbent activates 2 h at 200 ° C, argon protection, and air speed of 3000 ml h. -1 . Subsequent CO at 65 ° C 2 Gas adsorption (mixed gas CO 2 : N 2 = 15: 85), the speed is 3000ml h -1 The gas adsorbers detect the exhaust gas online. CO 2 The amount of adsorption is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com