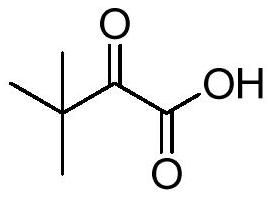
Preparation method of 3, 3-dimethyl-2-oxobutyric acid and triazinone
A technology of oxobutyric acid and dimethyl, applied in the field of pesticides, can solve problems such as being unfavorable to industrialized production, unable to be effectively recycled and reused, avoid high-salt wastewater and solid waste, reduce raw material costs, and achieve high yields. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] According to the first aspect of the present invention, there is provided a method for preparing 3,3-dimethyl-2-oxobutanoic acid, the method comprising: in the presence of a catalyst, at a pH of 7-13, containing oxygen The body is an oxidizing agent, and the step of oxidizing 3,3-dimethyl-2-hydroxybutyric acid and / or its salt.
[0024] According to the present invention, preferably, the oxygen-containing gas is oxygen and / or air. In view of improving reaction efficiency, the oxygen-containing gas is preferably oxygen. Considering cost reduction, the oxygen-containing gas is preferably air.
[0025] According to the present invention, preferably, the catalyst is one or more of ruthenium dioxide, Pt-C, Pd-C and ruthenium trichloride; more preferably, the catalyst is ruthenium dioxide, Pt One or more of -C and Pd-C. From the viewpoint of further improving the yield, ruthenium dioxide is more preferable.
[0026] Preferably, the catalyst is used in an amount of 3,3-dime...
Embodiment 1
[0056] 1) In a 500ml four-necked bottle, put 192g (0.32mol) of 20% by weight NaOH into it, heat up to 50°C under stirring, add 16.8g (0.1mol) of molten dichloropinatone dropwise at 50-70°C, drop After completion of the heat preservation reaction for 1 hour, GC analysis showed that the reaction was complete, the reaction solution was lowered to room temperature, adjusted to pH 2-3 with aqueous hydrochloric acid (concentration: 10% by weight), extracted with ethyl acetate, and the organic phase was removed to obtain 3,3- Dimethyl-2-hydroxybutyric acid (GC-MS M + =132) solid with a purity of 98% by weight and a yield of 95%.
[0057] 2) Put 13.5g of 3,3-dimethyl-2-hydroxybutyric acid into a 100ml autoclave, add 30% by weight of sodium hydroxide aqueous solution to completely dissolve, adjust the pH to 12, and add ruthenium dioxide (1mol%) 0.13g, stir and raise the temperature to 110°C, feed oxygen, keep the pressure at 4MPa, react for 8h, and GC analyzes that the reaction of raw...
Embodiment 2
[0059] 1) In a 500ml four-necked bottle, put 192g (0.32mol) of 20% by weight NaOH into it, heat up to 50°C under stirring, add 16.8g (0.1mol) of molten dichloropinatone dropwise at 50-70°C, drop After completion of the heat preservation reaction for 1 hour, GC analysis showed that the reaction was complete, the reaction solution was lowered to room temperature, adjusted to pH 2-3 with aqueous hydrochloric acid (concentration: 10% by weight), extracted with ethyl acetate, and the organic phase was removed to obtain 3,3- Dimethyl-2-hydroxybutyric acid solid, purity 98% by weight, yield 95%.
[0060] 2) Put 13.5g of 3,3-dimethyl-2-hydroxybutyric acid into a 100ml high-pressure reactor, add saturated potassium bicarbonate aqueous solution to completely dissolve, adjust the pH to 8, and add the catalyst ruthenium dioxide recovered in Example 1 , stirred and raised the temperature to 60°C, fed oxygen, kept the pressure at 6MPa, reacted for 8h, and the raw materials controlled in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com