Application of p-type D-A copolymer containing quinoxaline unit in efficient organic and perovskite solar cell
A solar cell, quinoxaline technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve problems such as insufficient photovoltaic performance, and achieve the effects of excellent photoelectric conversion efficiency, good phase separation, and improved aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
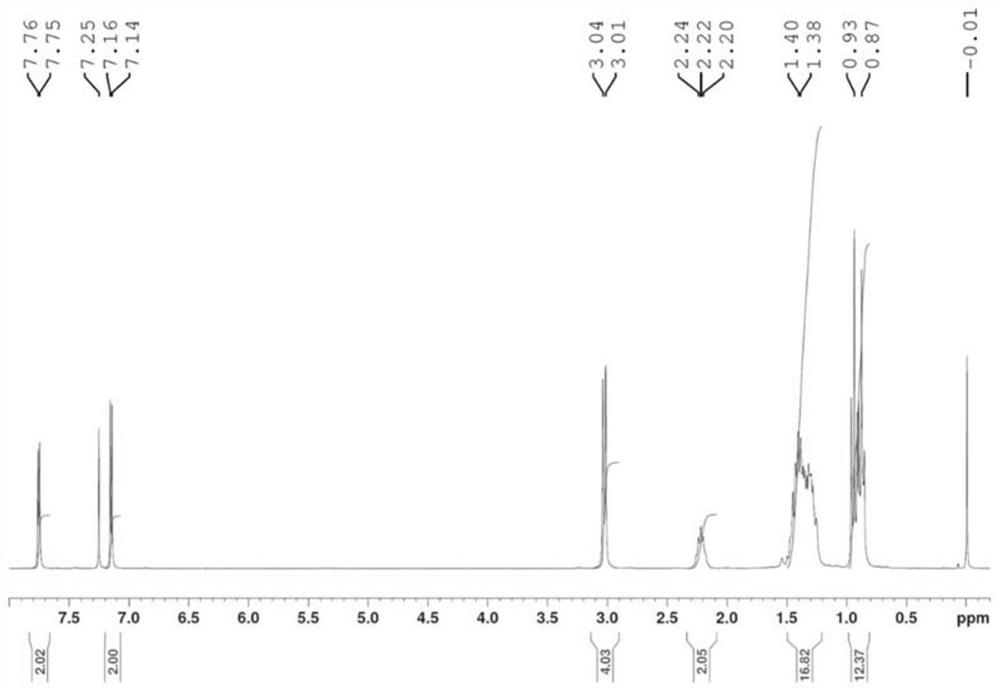
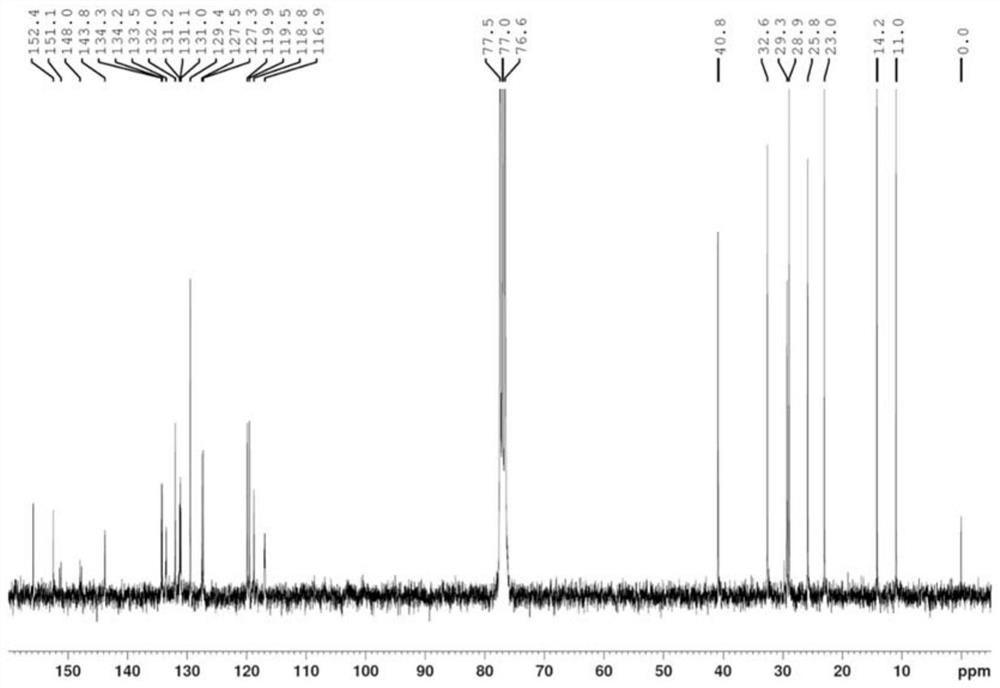
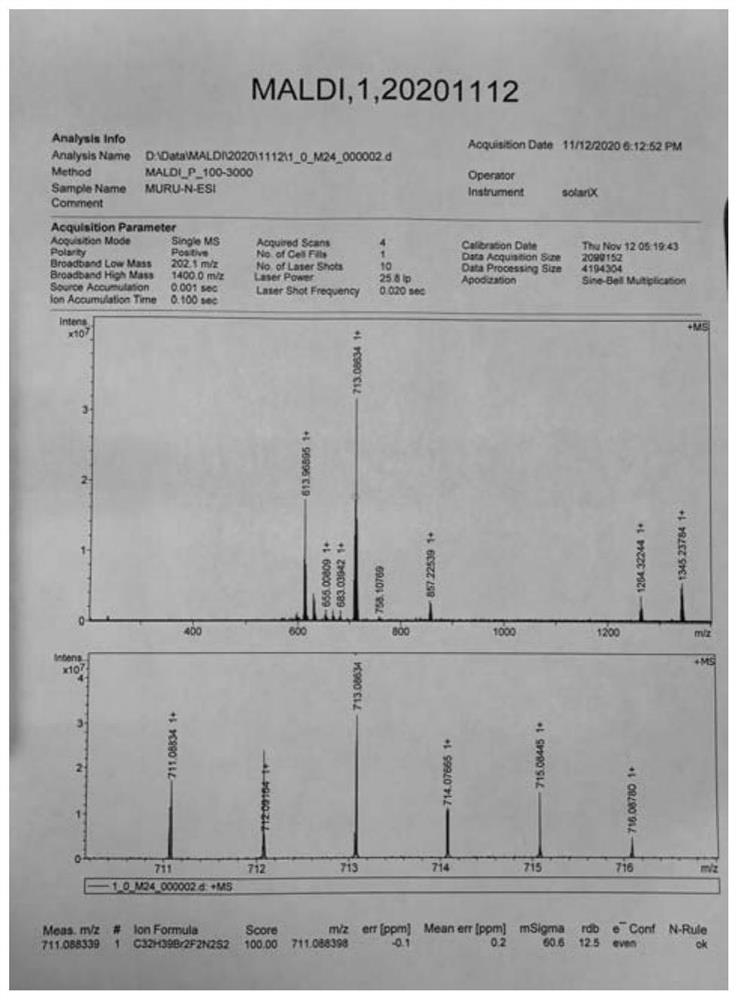
[0049] Embodiment 1: the preparation of quinoxaline polymer Z-1 (seeing the synthetic route below)
[0050] 1) Add the Grignard reagent of bromoisoctane (20mmol), CuBr (20mmol), LiBr (20mmol) and THF (30ml) into a two-necked flask, react at 0°C for half an hour, add oxalyl chloride (10mmol) After stirring overnight, Compound 2 was obtained in 30% yield.
[0051] 2) Dissolve compound 3 (5mmol) and 60ml ethanol in a 250ml single-necked bottle, and react at 0°C; add sodium borohydride (30mmol) in batches, and stir at 0°C for 5.5h to obtain compound 4 (3mmol), the yield 60%.
[0052] 4) Put compound 4 (3mmol) and 100ml of acetic acid into a three-necked flask, put compound 2 (3mmol) and 60ml of acetic acid into a constant pressure dropping funnel, drop them in at 60°C under Ar gas conditions, after dropping, 120 Reflux at ℃ for 2~3h, and then react at 90℃ overnight to obtain compound 5 (2 mmol), with a yield of 66.6%.
[0053] 5) Add compound 5 (2mmol), tributyl(thiophen-2-yl)s...
Embodiment 2
[0058] Embodiment 2: the preparation of quinoxaline polymer Z-2 (seeing the synthetic route below)
[0059] 1) Add the Grignard reagent of n-bromohexane (20mmol), CuBr (20mmol), LiBr (20mmol) and THF (30ml) into a two-neck flask, react at 0°C for half an hour, add oxalyl chloride (10mmol) and stir Overnight, Compound 9 was obtained in 35% yield.
[0060] 2) Dissolve compound 3 (6mmol) and 60ml ethanol in a 250ml single-necked bottle, and react at 0°C; add sodium borohydride (40mmol) in batches, and stir at 0°C for 5.5h to obtain compound 4 (3.6mmol). Rate 60%.
[0061] 4) Put compound 9 (3.5mmol) and 100ml of acetic acid into a three-neck flask, put compound 2 (3.5mmol) and 60ml of acetic acid into a constant pressure dropping funnel, drop them in at 60°C under Ar gas conditions, after the drops , refluxed at 120°C for 2-3h, and then reacted at 90°C overnight to obtain compound 10 (2.5mmol) with a yield of 71.4%.
[0062] 5) Add compound 5 (2.5mmol), tributyl(thiophen-2-yl)...
Embodiment 3
[0067] Embodiment 3: the preparation of quinoxaline polymer Z-3 (seeing the synthetic route below)
[0068] 1) Add brominated F-containing thienyl chain (20mmol), CuBr (20mmol), LiBr (20mmol) and THF (30ml) into a two-neck flask, react at 0°C for half an hour, add oxalyl chloride (10mmol) and stir Compound 15 was obtained in 25% yield overnight.
[0069] 2) Compound 3 (5mmol) was dissolved in 60ml ethanol in a 250ml single-necked bottle, and reacted at 0°C; sodium borohydride (40mmol) was added in batches, and stirred at 0°C for 5.5h to obtain compound 4 (3.2mmol). rate of 64%.
[0070] 4) Put compound 4 (2.5mmol) and 100ml of acetic acid into a three-neck flask, put compound 15 (2.5mmol) and 60ml of acetic acid into a constant pressure dropping funnel, drop them in at 60°C under Ar gas conditions, after the drops , refluxed at 120°C for 2-3h, and then reacted at 90°C overnight to obtain compound 16 (1.8mmol) with a yield of 72%.
[0071] 5) Add compound 16 (1.8mmol), tribu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com