Fibrilolytic enzyme as well as preparation method and application thereof
A technology for fibrinolytic and thrombolytic drugs, applied in the field of protease preparations, can solve the problems of unclear effect in vivo, complicated separation and purification methods, etc., and achieve the effects of good in vitro anticoagulation effect, suitable for mass production, and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 A kind of preparation method of fibrinase
[0051] 1. Carrier construction
[0052] (1) PCR amplification of SEQ ID NO.2: the amplification forward primer sequence is SEQ ID NO.3, and the reverse sequence is SEQ ID NO.4. The template is the cDNA obtained by reverse transcription after extracting the RNA of Cordyceps militaris. Among them, the Cordyceps militaris preservation number is CGMCC 3.14242; the RNA extraction kit was purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd., the article number is DP430; the reverse transcription kit was purchased from Takara, the article number is RR036A. The amplification program is: 95°C, 30s; 94°C, 30s, 55°C, 30s, 72°C, 90s, 35cycles; 72°C, 5min.
[0053](2) Ligation transformation: The PCR product was ligated with the pEASY-Blunt E2 vector. The pEASY-Blunt E2 vector was purchased from Beijing Quanshijin Biotechnology Co., Ltd., the product number was CE211, the reaction temperature was 25°C, and the r...
Embodiment 2
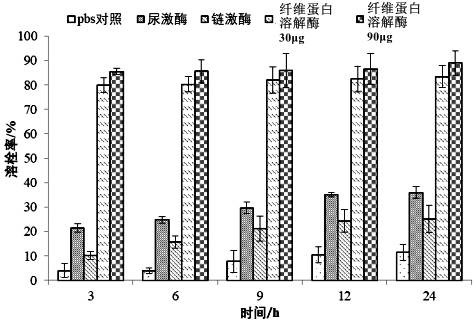
[0060] Embodiment 2 A kind of thrombolytic experiment of fibrinase
[0061] In this embodiment, the in vitro thrombolytic activity of the fibrinolytic enzyme obtained in Example 1 is measured, and the specific methods and steps are as follows:
[0062] Heart blood was collected from healthy SD rats (purchased from Beijing Weitong Lihua Co., Ltd.), divided into 0.6 mL tubes, and the empty tubes of centrifuge tubes were recorded as m1. After the blood was left to coagulate naturally, centrifuge at 3000rpm for 10min to remove the supernatant serum, blot the liquid with filter paper, weigh the mass of the remaining blood clot and the centrifuge tube, and record it as m2.
[0063] Experiments were carried out according to the following groups, with 3 parallels in each group:
[0064] (1) 200 μL of 1×PBS buffer;
[0065] (2) 200 μL of 0.15 g / L urokinase;
[0066] (3) 200 μL of 0.15 g / L streptokinase;
[0067] (4) 0.15g / L fibrinolytic enzyme 200μL;
[0068] (5) 200 μL of 0.45 g / ...
Embodiment 3
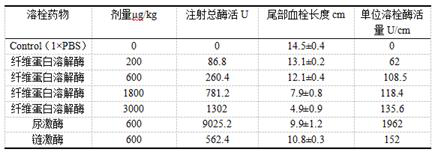
[0073] Embodiment 3 A kind of thrombolytic experiment of fibrinase
[0074] Determination of in vivo thrombolytic activity of recombinant enzyme (effect on rat tail vein thrombosis):
[0075] The rat tail vein thrombosis model was used to study the thrombolytic activity of the fibrinolytic enzyme obtained in Example 1 in rats. Select 1.2 mg / kg concentration of k-carrageenan to inject into the tail vein of rats, observe the formation of tail thrombus after 24 hours, and measure the length of the formed wine red thrombus.
[0076] Forty-two Wistar male rats (purchased from Beijing Weitong Lihua Co., Ltd.) were injected with equal volumes in the following groups:
[0077] A. Inject 1×PBS;
[0078] B. Injection of 200 μg / kg fibrinolytic enzyme;
[0079] C. Injection of 600 μg / kg fibrinolytic enzyme;
[0080] D. Injection of 1800 μg / kg fibrinolytic enzyme;
[0081] E. Injection of 3000 μg / kg fibrinolytic enzyme;
[0082] F. Inject 600 μg / kg urokinase;
[0083] G. Inject 600 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com