Preparation method of 2-trifluoromethyl substituted quinazolinone compound and application of 2-trifluoromethyl substituted quinazolinone compound in synthesis of pharmaceutical molecules
A technology of trifluoromethyl and quinazolinone, which is applied in the field of organic synthesis, can solve the problems of expensive reaction substrates, harsh reaction conditions, and narrow substrate range, and achieve simple post-treatment, easy operation, and high reaction efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention will be further described below in conjunction with specific embodiments.
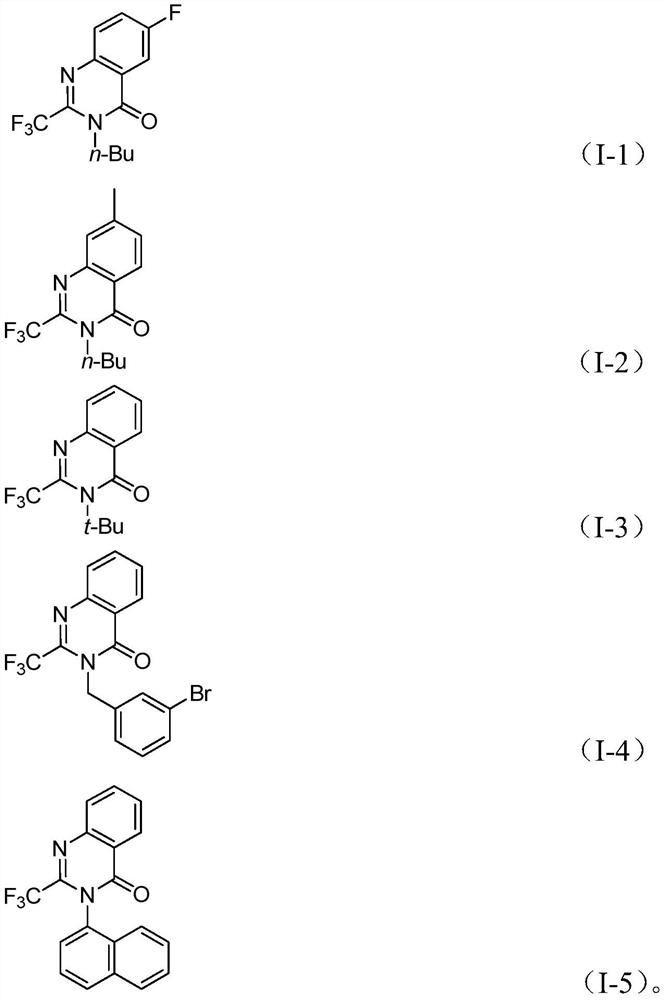
[0033] Add palladium trifluoroacetate, triphenylphosphine, TFBen, sodium carbonate, trifluoroethylimidoyl chloride (II), amine (III) and 2 mL of organic solvent into a 35 mL Schlenk tube according to the raw material ratio in Table 1, and mix Stir evenly, react for 16-30 hours according to the reaction conditions in Table 2, filter, mix the sample with silica gel, obtain the corresponding 2-trifluoromethyl-substituted quinazolinone compound (I) through column chromatography purification, and the reaction process is as follows: Shown:
[0034]
[0035] The raw material addition of table 1 embodiment 1~15
[0036]
[0037] Table 2
[0038]
[0039]
[0040] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Ph is phenyl, Bn is benzyl, Me is methyl, OMe is methoxy, n-Bu is n-butyl, t-Bu is tert-butyl, i-Pr is isopropyl, Cy is cyclohex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com