Golgi apparatus and genetic engineering exosome hybrid membrane coated retinoic acid in-situ spray hydrogel vaccine, and preparation method and application thereof
A technology of genetic engineering and Golgi apparatus, applied in the suppression and/or treatment of postoperative recurrence of melanoma, in the field of retinoic acid in situ spray hydrogel vaccine, can solve the problem of low response rate of immunotherapy, recurrence of patients, etc. problem, to achieve the effect of prolonging the survival time and reducing the recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
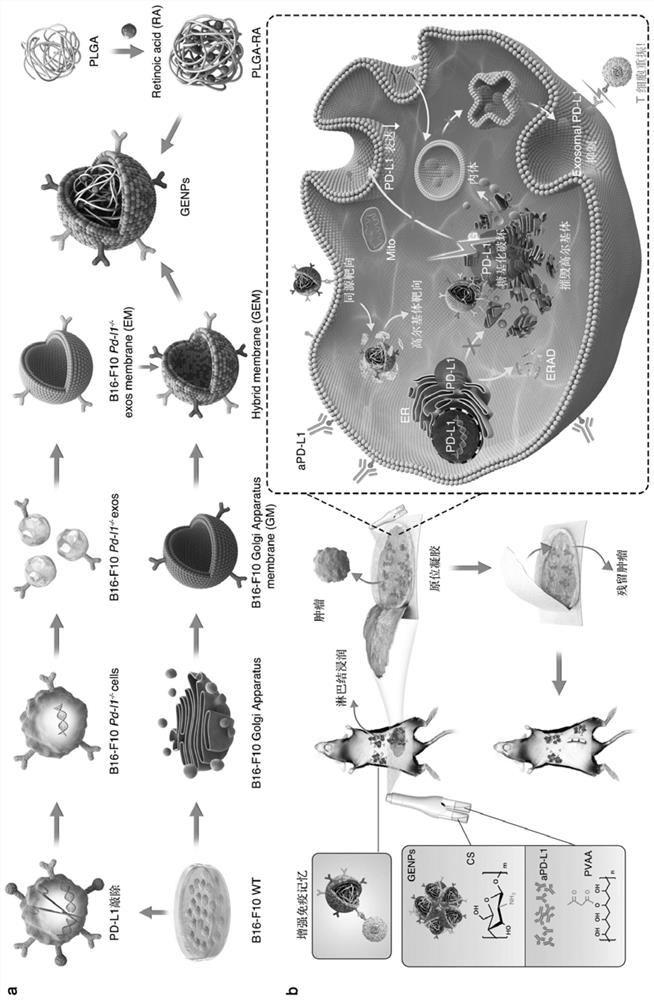
[0126] A method for preparing retinoic acid biomimetic nanoparticles (GENPs) coated with hybrid membranes of Golgi apparatus and genetically engineered exosomes, comprising the following steps:
[0127] The preparation details of GENPs, such as figure 1 shown.
[0128] Step 1: preparing nanoparticles of retinoic acid;
[0129] Retinoic acid-loaded PLGA nanoparticles were prepared by the oil-in-water (O / W) emulsification solvent evaporation method. Dissolve 60 mg of PLGA and 2 mg of retinoic acid in 4 mL of dichloromethane. The organic phase was transferred to 8 mL of 1.5% PVA solution. Then, the solution was sonicated at 400 W for 5 minutes in an ice bath. The emulsion was then shaken overnight at room temperature to eliminate the organic solvent and form uniformly dispersed nanoparticles. Subsequently, the particle suspension was centrifuged at 16000g for 20 minutes. The prepared nanoparticles were then washed three times with an equal volume of deionized water.
[013...
Embodiment 2
[0142] Prepare a dosage form, which is retinoic acid biomimetic nanoparticles coated with Golgi apparatus and genetically engineered exosome hybrid membrane, and loaded with PVAA / CS gel (aPD-L1@GENPs@Gel);
[0143] The method of Example 1 was used to prepare retinoic acid biomimetic nanoparticles GENPs coated with hybrid membranes of Golgi apparatus and genetically engineered exosomes; the obtained GENPs and PD-L1 monoclonal antibodies were loaded into PVAA and CS respectively to obtain in situ spray Hydrogel vaccine, which inhibits the secretion of exosome PD-L1, revitalizes lymph node immune cells to trigger immune memory and then activates the Golgi apparatus of the systemic immune response and the retinoic acid biomimetic hydrogel coated with the hybrid membrane of genetically engineered exosomes vaccine. Specifically:
[0144] Dry PVA powder (5.0 g) was added to a three-necked flask containing 45 mL DMSO. The mixture was heated to 90°C under magnetic stirring to complet...
Embodiment 3
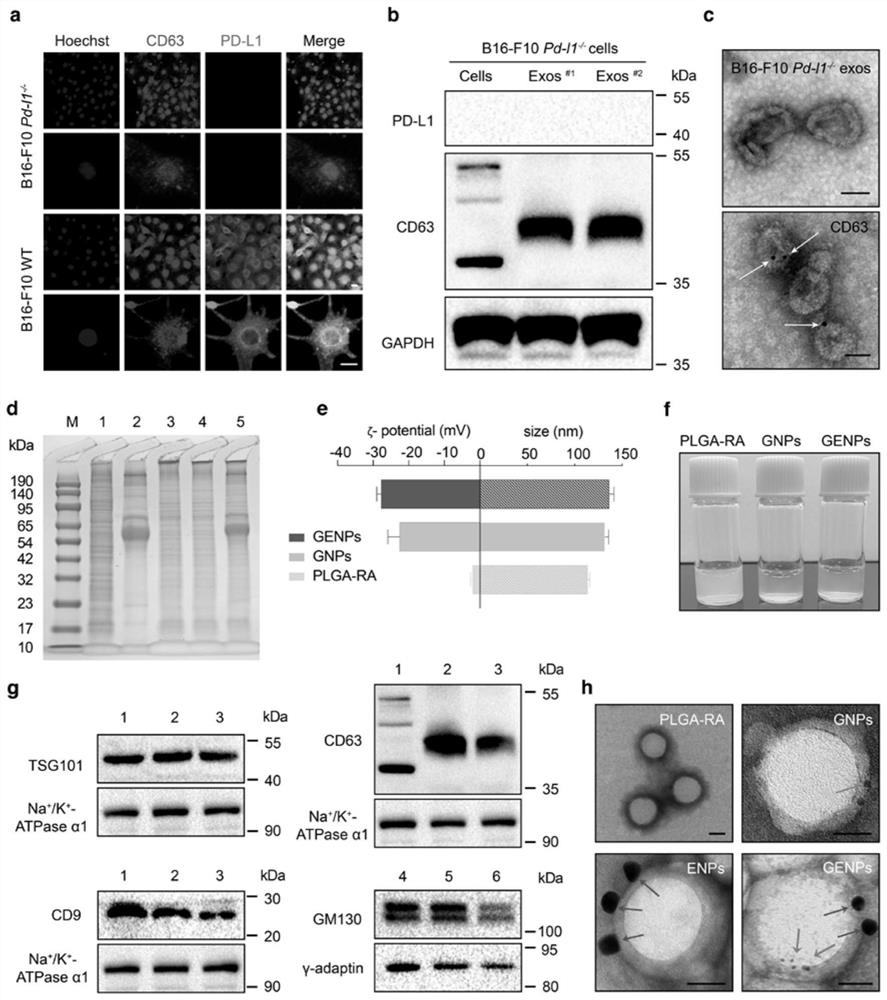
[0147] Golgi-disrupting ability of retinoic acid biomimetic nanoparticles (GENPs) coated with hybrid membrane of genetically engineered exosomes and exosomal PD-L1 inhibitory ability
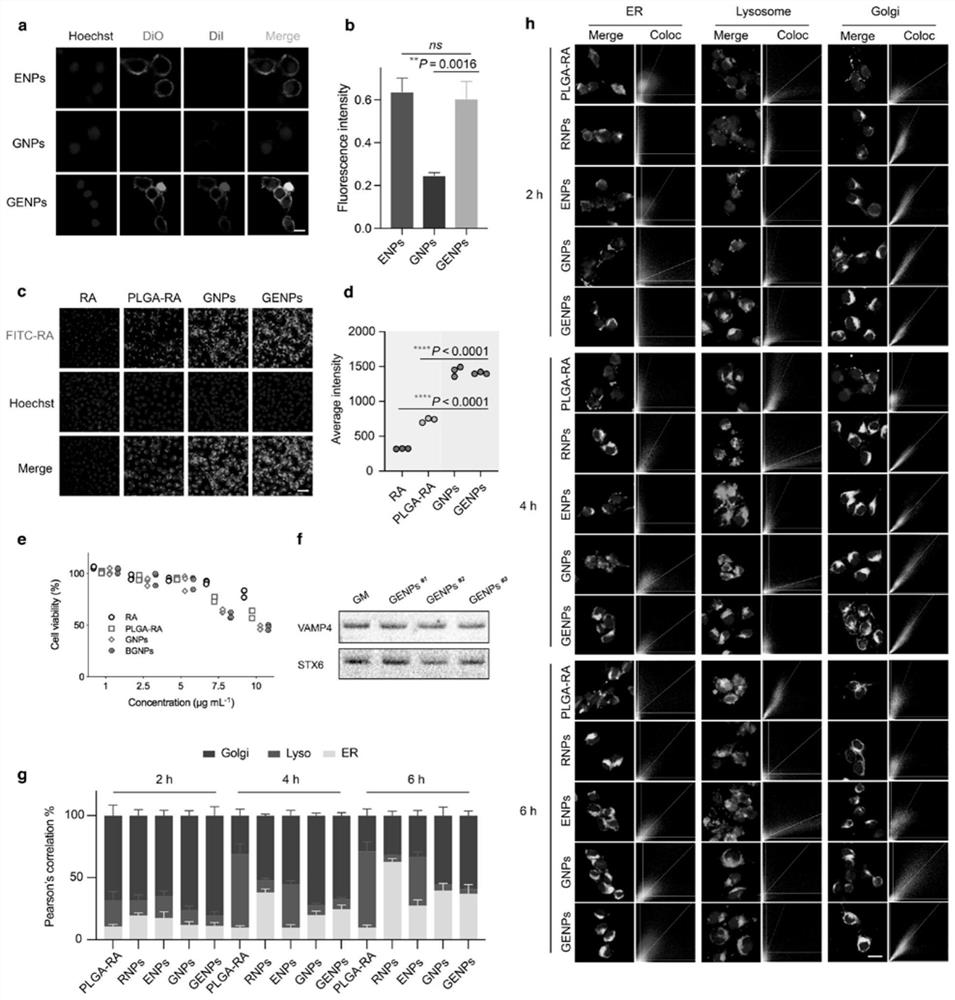
[0148] To assess the cell adhesion of GENPs, B16-F10 cells were incubated with fluorescently labeled nanoparticles for 1 h at 4 °C. Such as image 3 As shown in a and 3b, although both GENPs and ENPs showed high fluorescence signals on B16-F10 cells, GNPs showed weak adhesion to B16-F10 cells, indicating that EM coating promoted the adhesion of nanoparticles to tumor cells. affinity.
[0149] Confocal microscopy was used to study the cellular internalization of GENPs. B16-F10 cells were incubated with FITC-RA, PLGA-RA, GNPs and GENPs for 6 hours, respectively. Such as image 3 As shown in c, various intensities of fluorescence were observed in the cytoplasm. In B16-F10 cells, both GNPs and GENPs showed high intracellular fluorescence of FITC-RA ( image 3 d), which indicates that the enhan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com