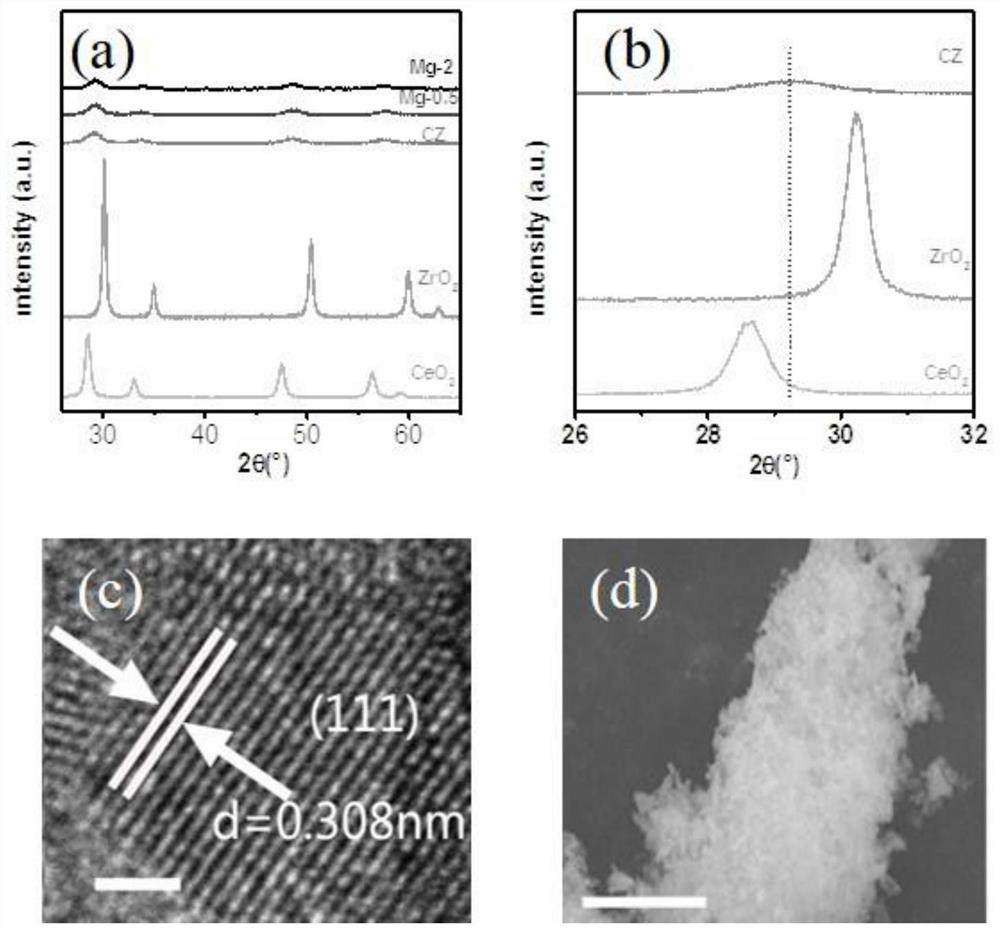
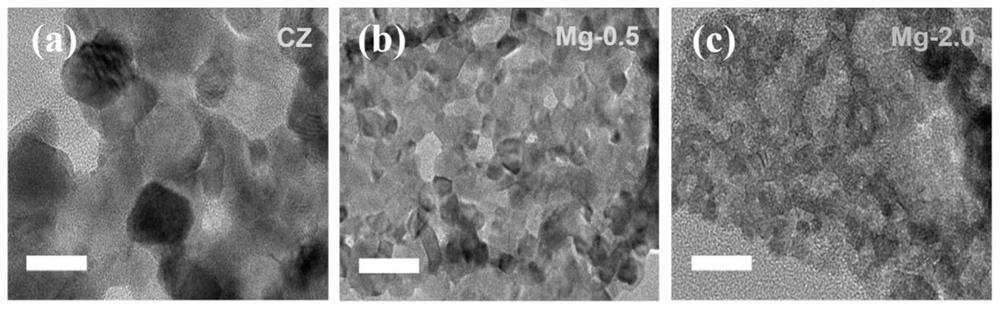
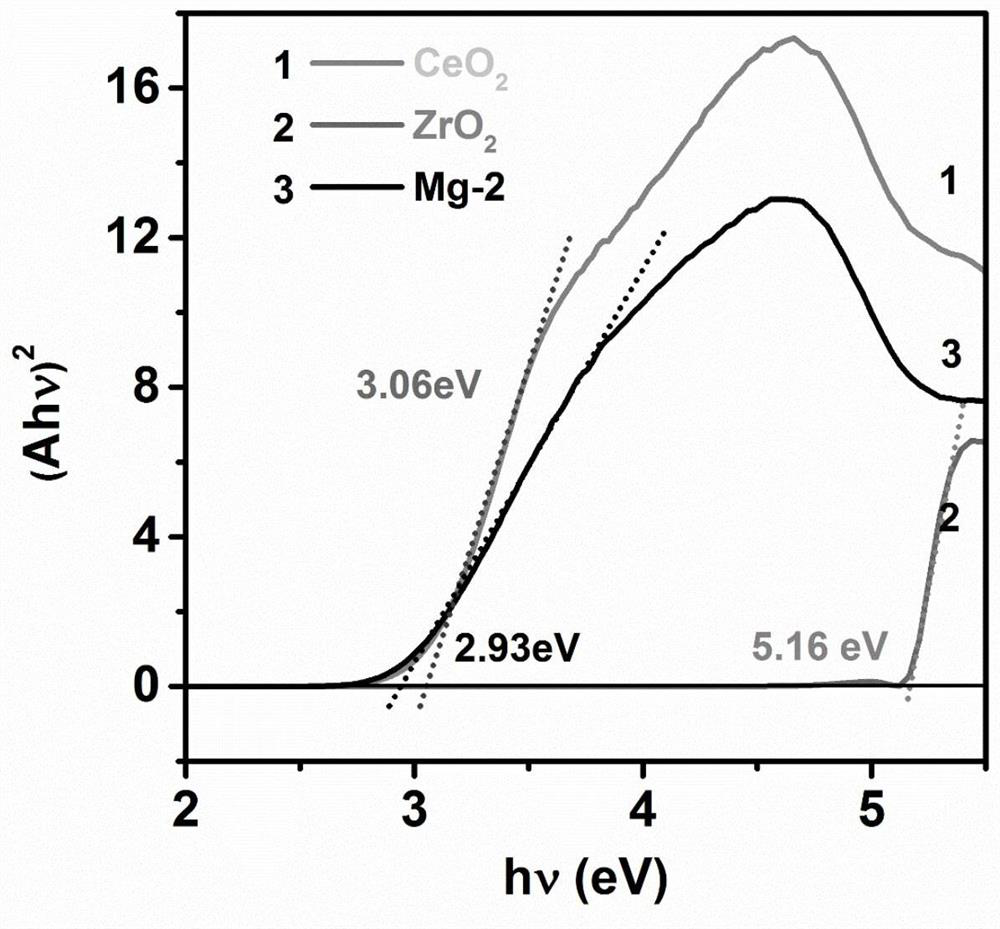
Highly stable defect state cerium-zirconium double metal oxide catalyst, preparation method and application thereof
A dual-metal oxide and defect state technology, which is applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve kinetic difficulties, complex operations, and energy consumption High-level problems, to achieve high-efficiency methane dehydrogenation coupling, high atom utilization, and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation method of cerium-zirconium double metal oxides with different cerium-zirconium ratios and its application in photocatalytic methane dehydrogenation coupling reaction
[0038] (1) Preparation of cerium-zirconium double metal oxide: Weigh commercial cerium nitrate hexahydrate, zirconium oxynitrate hydrate, and glycine in a small beaker with a molar ratio of 3:7:10. After uniformity, the solution was transferred to an evaporating dish, evaporated to dryness at 90°C, and then heated to 170°C, the mixture released heat and spontaneously ignited to obtain a light yellow sample of cerium-zirconium double metal oxide, marked as C 3 Z 7 ; Afterwards, the catalyst is used in the methane dehydrogenation coupling reaction.
[0039] (2) Weigh 50 mg of catalyst into a 10 mL small beaker, disperse it evenly with acetone, place it in a 70°C oven to evaporate the solvent, and obtain a catalyst with a uniform surface, put the catalyst into a quartz reactor, and pl...
Embodiment 2
[0040] Example 2: Preparation method of cerium-zirconium double metal oxides with different cerium-zirconium ratios and its application in photocatalytic methane dehydrogenation coupling reaction
[0041] (1) Preparation of cerium-zirconium double metal oxide: Weigh 4 mmol of commercial cerium nitrate hexahydrate, zirconium oxynitrate hydrate, and glycine in a molar ratio of 4:6:10 in a small beaker, add 10 mL of deionized water, and stir magnetically After uniformity, the solution was transferred to an evaporating dish, evaporated to dryness at 90°C, and then heated to 170°C, the mixture released heat and spontaneously ignited to obtain a light yellow sample of cerium-zirconium double metal oxide, marked as C 4 Z 6 ; Afterwards, the catalyst is used in the methane dehydrogenation coupling reaction.
[0042] (2) Weigh 50 mg of catalyst into a 10 mL small beaker, disperse it evenly with acetone, place it in a 70°C oven to evaporate the solvent, and obtain a catalyst with a uni...
Embodiment 3
[0043] Example 3: Preparation method of cerium-zirconium double metal oxides with different cerium-zirconium ratios and its application in photocatalytic methane dehydrogenation coupling reaction
[0044] (1) Preparation of cerium-zirconium double metal oxide: Weigh commercial cerium nitrate hexahydrate, zirconium oxynitrate hydrate, and glycine in a small beaker with a molar ratio of 5:5:10. After uniformity, the solution was transferred to an evaporating dish, evaporated to dryness at 90°C, and then heated to 170°C, the mixture released heat and spontaneously ignited to obtain a light yellow sample of cerium-zirconium double metal oxide, labeled as CZ; after that, the catalyst was used for methane removal Hydrogen coupling reaction.
[0045] (2) Weigh 50 mg of catalyst into a 10 mL small beaker, disperse it evenly with acetone, place it in a 70°C oven to evaporate the solvent, and obtain a catalyst with a uniform surface, put the catalyst into a quartz reactor, and place the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com