Method for determining related substances in mecobalamin tablet
A technology for methylcobalamin tablets and related substances, applied in the field of drug analysis, can solve problems such as hidden dangers in product quality control, certain hidden dangers in product quality control, limit control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
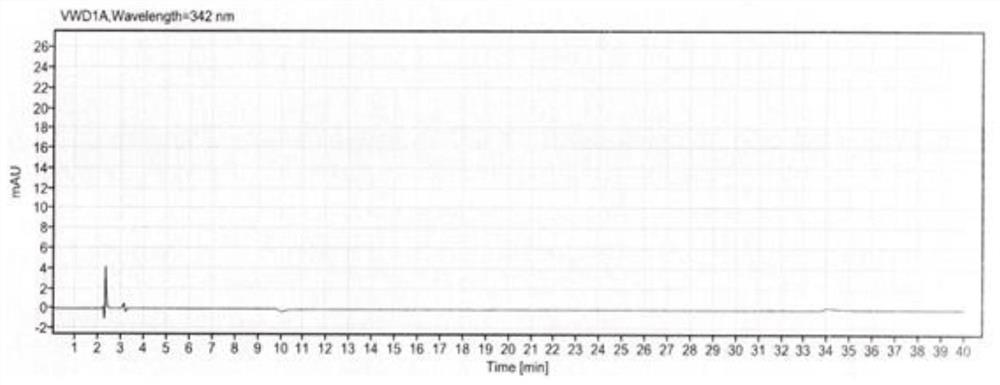
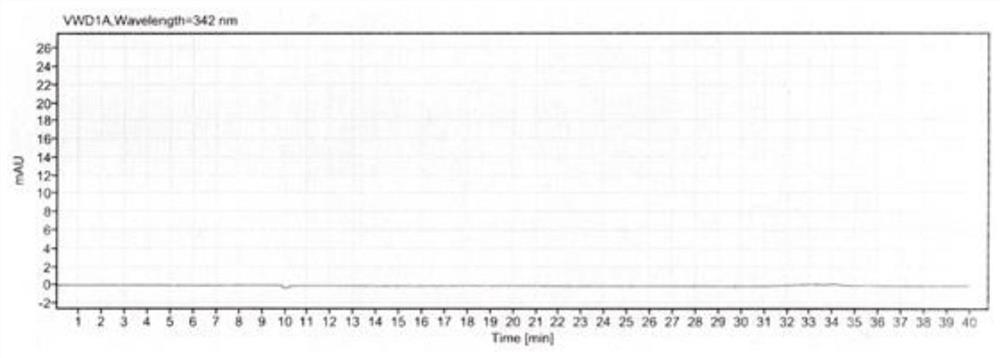
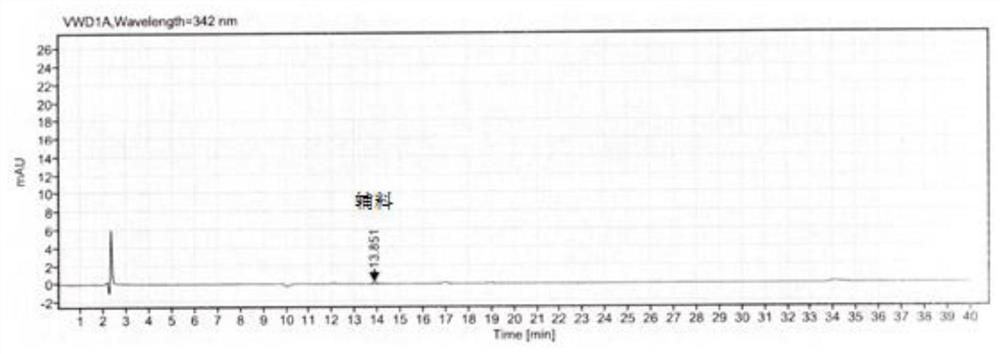
[0050]In order to better understand the purpose, structure and function of the present invention, a method for the determination of related substances in methylcobalamin provided by the present invention will be described in further detail below in conjunction with the accompanying drawings.
[0051] A method for assaying related substances in methylcobalamin, comprising the steps of:
[0052] S1 prepares mobile phase, comprises the following steps:
[0053] Potassium dihydrogen phosphate solution: Weigh an appropriate amount of potassium dihydrogen phosphate, add pure water to dissolve, mix well, adjust the pH value with sodium hydroxide solution or phosphoric acid solution, and filter with a water-based filter membrane to obtain potassium dihydrogen phosphate solution.
[0054] Mobile phase A: Take potassium dihydrogen phosphate solution and acetonitrile, mix them in proportion, and degas them by ultrasonic to obtain mobile phase A.
[0055] Mobile phase B: Mix acetonitrile...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com