Preparation method of (R)-3-palmitoyl oxybutyric acid
A technology of palmitoyloxybutyric acid and tert-butyl palmitoyloxybutyrate, which is applied in the field of preparation of -3-palmitoyloxybutyric acid, can solve the problems of acidosis and difficult penetration of the blood-brain barrier, etc. Achieve the effects of simple route, easy industrial production, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
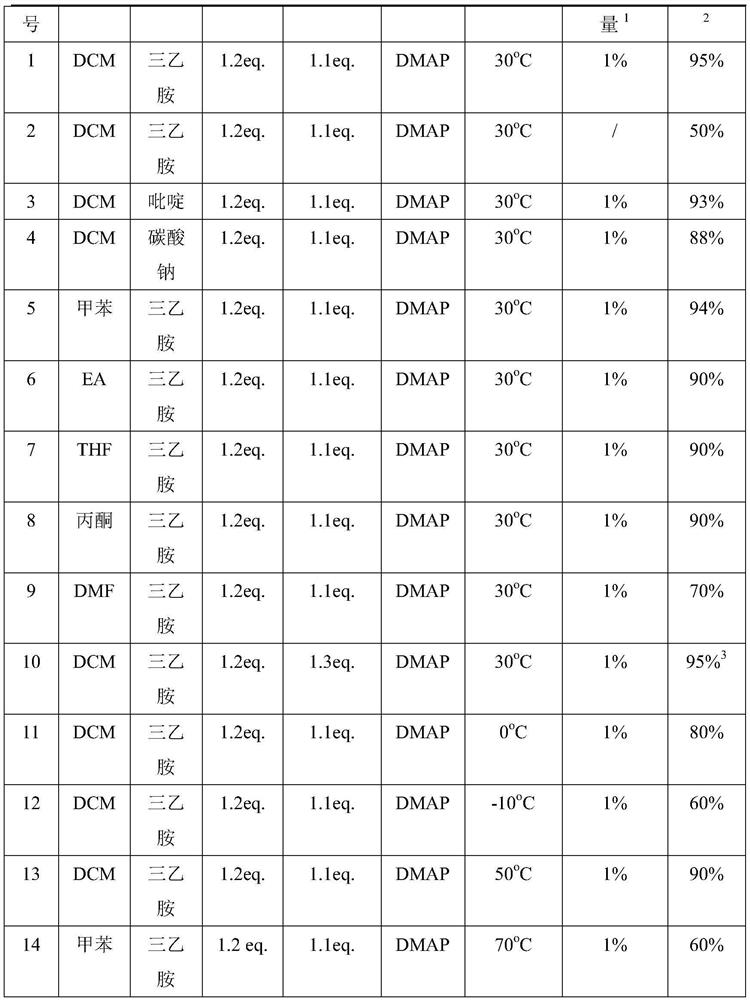
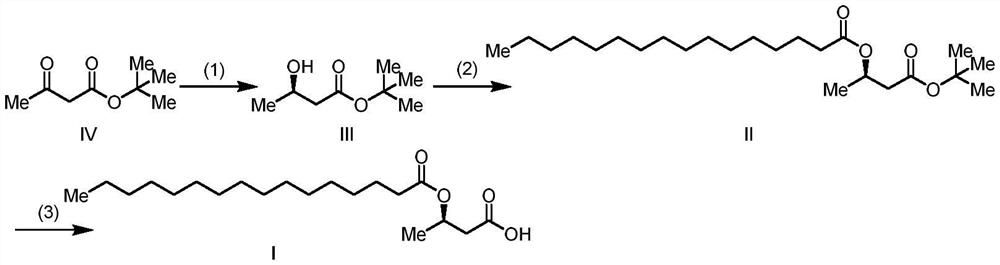
[0045] Embodiment 1: the preparation of (R)-3-palmitoyloxybutyric acid
[0046]
[0047] Add 200g of tert-butyl acetoacetate to the autoclave, add 200mL of methanol, and add 400mg of (R)-BINAP-Ru. Nitrogen replacement three times, hydrogen replacement three times. React under 3MPa at 35-45°C for 16 hours, and monitor the completion of the reaction. Methanol was distilled off under reduced pressure. The residue was distilled under reduced pressure to obtain 184 g of tert-butyl (R)-3-hydroxybutyrate as a colorless oil. Yield: 92%, chemical purity: 99%, optical purity: 99%.
[0048]100g (R)-tert-butyl 3-hydroxybutyrate was placed in a 2L flask, and 600mL of dichloromethane, 76g (1.2eq.) of triethylamine, and 1g of DMAP were added. 189g of hexadecanoyl chloride (1.1eq.) was added at 15-25°C. Keep warm for 10-16 hours, and monitor the completion of the reaction. The reaction solution was suction filtered, the filtrate was added with water, washed and separated, and the org...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com