Preparation method of high-purity amino sugar drug impurity
A technology of amino sugar and purification method, applied in the field of medicine, can solve the problems of unfriendly environment, cumbersome process, application limitation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
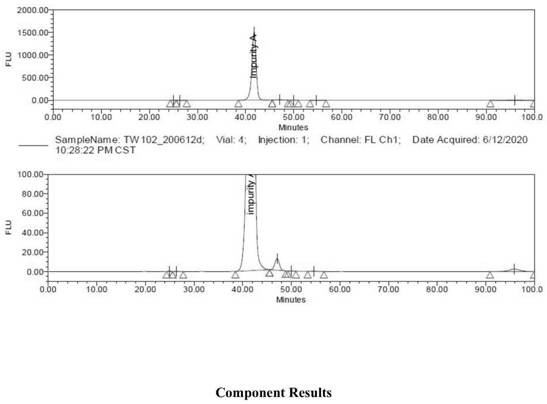
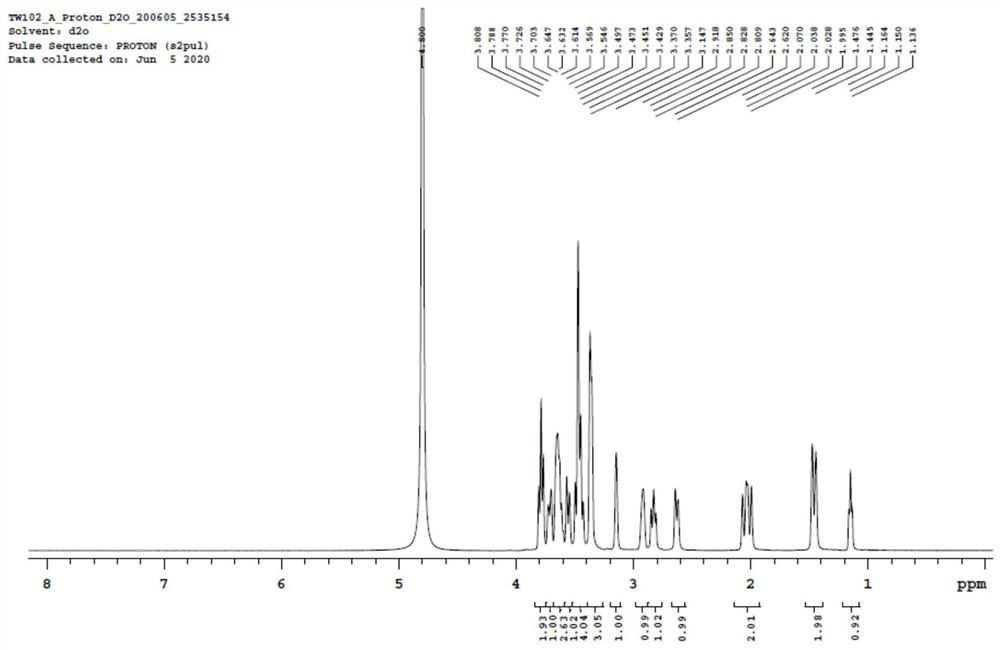
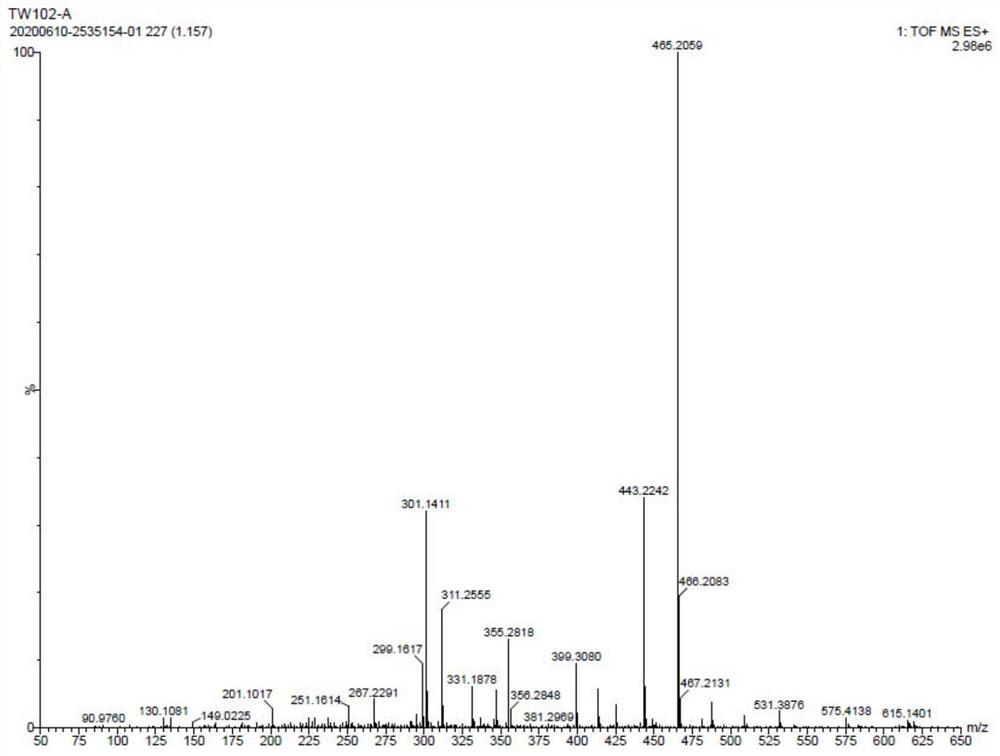
[0067] Add 5.0g of mycophenolamine, 0.5g of 1,3-dihydroxyacetone (0.2 equivalent), N,N-dimethylformamide (240ml) into a stirred three-neck flask, and stir for 5-10min. After adding 0.2 g of tartaric acid, 2.5 g of sodium triacetylborohydride was added in three batches. After 10 minutes, it was heated to 40°C for 10 hours. After the reaction, the HPLC purity of the aminosugar drug impurity in the reaction product was 20%.
Embodiment 2
[0069] Add 5.0 g of mycosamine, 1.4 g of 1,3-dihydroxyacetone (0.6 equivalent), and dimethyl sulfoxide (240 ml) into the reaction flask, and stir for 5 minutes to dissolve it. Then add 0.3 g of formic acid, stir for 1 min, and add 2.4 g of sodium borohydride in five batches. After reacting for 20min, heat to 80°C and react for 1h. After the reaction, the HPLC purity of the aminosugar drug impurity in the reaction product was 28%.
Embodiment 3
[0071] Add 5.0 g of mycophenolamine, 2.3 g of 1,3-dihydroxyacetone (1.0 equivalent), and acetonitrile (240 ml) into the stirred reaction flask, and stir to dissolve it. Add 0.3g of citric acid, stir for 60min, and add 0.8g of zinc powder. After 10 minutes, it was heated to 45°C for 8 hours. After the reaction, the HPLC purity of the aminosugar drug impurity in the reaction product was 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com