Stent for keratoprosthesis and preparation method thereof
A technology of artificial cornea and chondrocytes, applied in the field of artificial cornea support and its preparation, to achieve the effect of reducing discharge and water leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Chondrocyte Extracellular Matrix / Bone Matrix-Adipose Mesenchymal Stem Cells (DCECM / DBM-rADMSCs) Composite Scaffold
[0039] 【Preparation of Chondrocyte Extracellular Matrix / Bone Matrix (DCECM / DBM) Scaffold】
[0040] 1-1 Preparation of chondrocyte extracellular matrix (DCECM)
[0041] (1) Take out 100 g of fetal bovine articular cartilage one day old, and wash it with sterile distilled water for 3 times, each time for 10 minutes;
[0042] (2) Soak in 1% acetic acid for 3 times, each time for 3 hours;
[0043] (3) Hypotonic freezing and thawing in a freezer at -20°C for 8 hours;
[0044] (4) Melt ice, discard water and wash with distilled water twice;
[0045] (5) soak and sterilize in 3% hydrogen peroxide solution for 6 hours;
[0046] (6) Washing with sterile three-distilled water for 3 times, each time for 10 minutes;
[0047](7) Pulverize in a pulverizer to a homogenate with cells in millimeter, micron, and nanometer scales;
[0048] (...
Embodiment 2
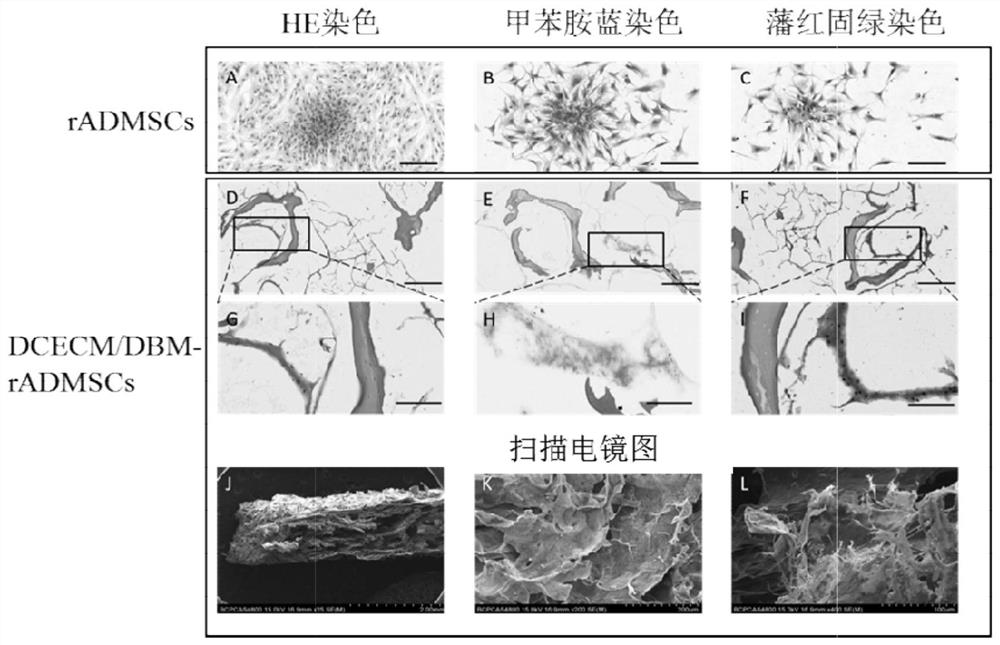
[0078] Example 2 Histopathological examination of chondrocyte extracellular matrix / bone matrix-rabbit adipose-derived mesenchymal stem cells (DCECM / DBM-rADMSCs) composite scaffold
[0079] 2-1 Prepare chondrocyte extracellular matrix / bone matrix (DCECM / DBM) scaffold in the same manner as in Example 1
[0080] 2-2 Primary culture of rabbit adipose-derived mesenchymal stem cells (rADMSCs)
[0081] (1) Sumianxin + ketamine (1:1) is anesthetized by intramuscular injection of 0.3ml / kg;
[0082] (2) Put the rabbit on the operating table, remove the hair in the operation area, disinfect the operation field with iodophor cotton ball three times, and wipe with alcohol three times;
[0083] (3) Make an incision about 3 cm long in the right lower groin of the rabbit, the depth reaches the subcutaneous tissue, bluntly separate the subcutaneous tissue, explore while separating until the subcutaneous fat is found, carefully cut out the blocky fat tissue, and pay attention to avoiding l...
Embodiment 3
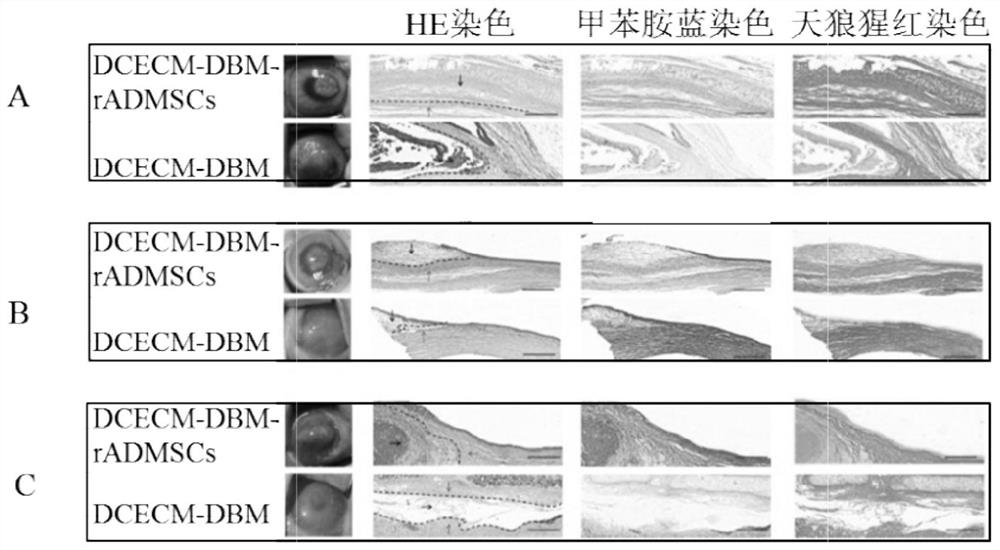
[0100] Example 3 Animal experiment of cartilage generation effect of chondrocyte extracellular matrix / bone matrix-rabbit adipose-derived mesenchymal stem cells (DCECM / DBM-rADMSCs) composite scaffold
[0101] 3-1 Prepare chondrocyte extracellular matrix / bone matrix-rabbit adipose-derived mesenchymal stem cells (DCECM / DBM-rADMSCs) composite scaffold
[0102] 3-2 Rabbit corneal chondrocyte extracellular matrix / bone matrix-rabbit adipose-derived mesenchymal stem cells (DCECM / DBM- rADMSCs) composite scaffold autografting
[0103] (1) Lu Mianxin: Midazolam = 1:1, 0.2ml / Kg intramuscular injection for anesthesia;
[0104] (2) Place the rabbit on the operating table, sterilize the right eye with iodophor, deiodine with alcohol, spread a sterile drape, place a lid speculum, and treat the eye with Benuoxi;
[0105] (3) scrape off the corneal epithelium with a corneal epidermectomy;
[0106] (4) Make a lamellar incision about 3 mm long at a distance of 2 mm from the corneal limbu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com