Preparation method of sulfobetaine type waterborne polyurethane chain extender
A sulfobetaine type, water-based polyurethane technology, applied in the preparation of sulfonic acid, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of difficult reuse, difficult temperature control, low product yield, etc. The production cycle is time-consuming, not easy to agglomerate, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
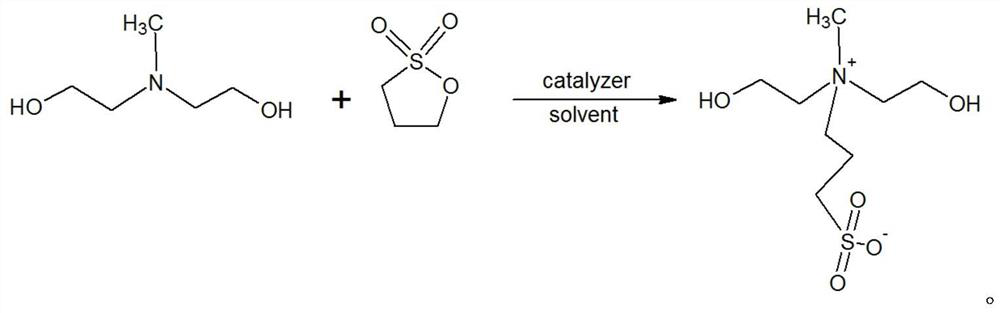
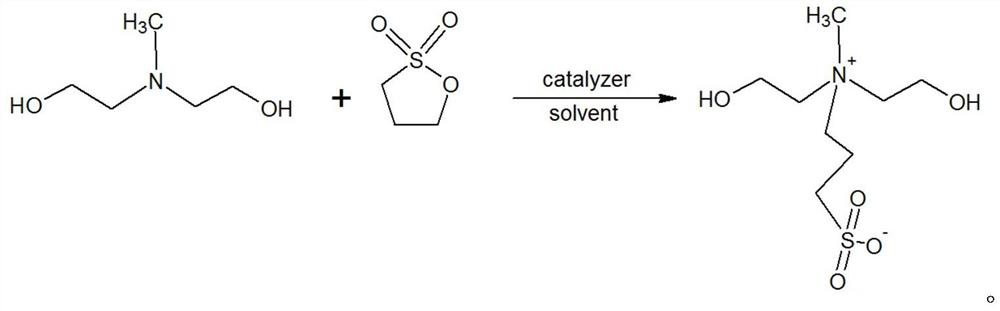
[0029] A kind of N, the preparation method of N-bis(2-hydroxyethyl)-N-methyl-3-sulfonate-1-propylamine, its steps are as follows:
[0030] (1) Add 107.4g of N-methyldiethanolamine into a 1000ml four-necked flask equipped with a thermometer and tetrafluoro stirring, add 280g of anhydrous methanol and stir to dissolve. After complete dissolution, 1 g of powdered potassium hydroxide solid was added. 100g of 1,3-propane sultone is added dropwise under the condition of temperature control at 30-50°C, the dropping time is 1-3h, and the aging time is 1-2h. Two consecutive samples were taken, and the tertiary amine content of the central control sample was calculated to be 2.83% and 2.81% respectively as raw material N-methyldiethanolamine residues. The suspension was cooled to below 20°C, and 247.6g of crude MDAPS was obtained by filtration.
[0031] (2) Use 50ml of methanol to rinse the MDAPS crude product, repeat 2 to 3 times, detect that the sample is basically free of tertiary a...
Embodiment 2
[0033] A kind of N, the preparation method of N-bis(2-hydroxyethyl)-N-methyl-3-sulfonate-1-propylamine, its steps are as follows:
[0034] (1) 102.4g of N-methyldiethanolamine was added to a 1000ml four-neck flask equipped with a thermometer and PTFE stirring, and 280g of methanol recovered in Example 1 was added and stirred to dissolve. After complete dissolution, 1 g of powdered potassium hydroxide solid was added. 100g of 1,3-propane sultone is added dropwise under the condition of temperature control at 30-50°C, the dropping time is 1-3h, and the aging time is 1-2h. Sampling was taken twice in a row, and the tertiary amine content of the central control sample was calculated to be 3.51% and 3.49% respectively as raw material N-methyldiethanolamine residues. The suspension was cooled to below 20°C, and 253.1 g of crude MDAPS was obtained by filtration.
[0035] (2) Rinse the crude MDAPS with 50ml of fresh methanol, repeat 2 to 3 times, detect that the sample is basically f...
Embodiment 3
[0037] A kind of N, the preparation method of N-bis(2-hydroxyethyl)-N-methyl-3-sulfonate-1-propylamine, its steps are as follows:
[0038] (1) Add 356.2g of N-methyldiethanolamine into a 3000ml four-neck flask equipped with a thermometer and PTFE stirring, add 800g of cyclohexane and stir to dissolve. After complete dispersion and dissolution, 5.3 g of potassium methoxide was added. 304.3g of 1,3-propane sultone was added dropwise under the condition of temperature control at 30-50°C, the dropping time was 1-3h, and the aging time was 1-2h. Sampling was taken twice in a row, and the tertiary amine content of the central control sample was calculated to be 4.70% and 4.68% respectively as raw material N-methyldiethanolamine residues. The suspension was cooled to below 20°C, and 732.7g of crude MDAPS was obtained by filtration.
[0039] (2) Use 160ml of fresh cyclohexane to wash the crude MDAPS, repeat 2 to 3 times, detect that the sample has basically no tertiary amine content,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com