Non-fullerene acceptor compound containing benzoselenadiazole and organic optoelectronic element comprising the same
A non-fullerene acceptor, benzoselenodiazole technology, applied in the field of compounds, can solve the problems of difficult development of non-fullerene acceptor compounds, difficult control of compound types, low power conversion efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of compound N1, N2
[0069]
[0070] Compounds M1 to M7 were synthesized as described below
[0071] a. Synthesis of M1
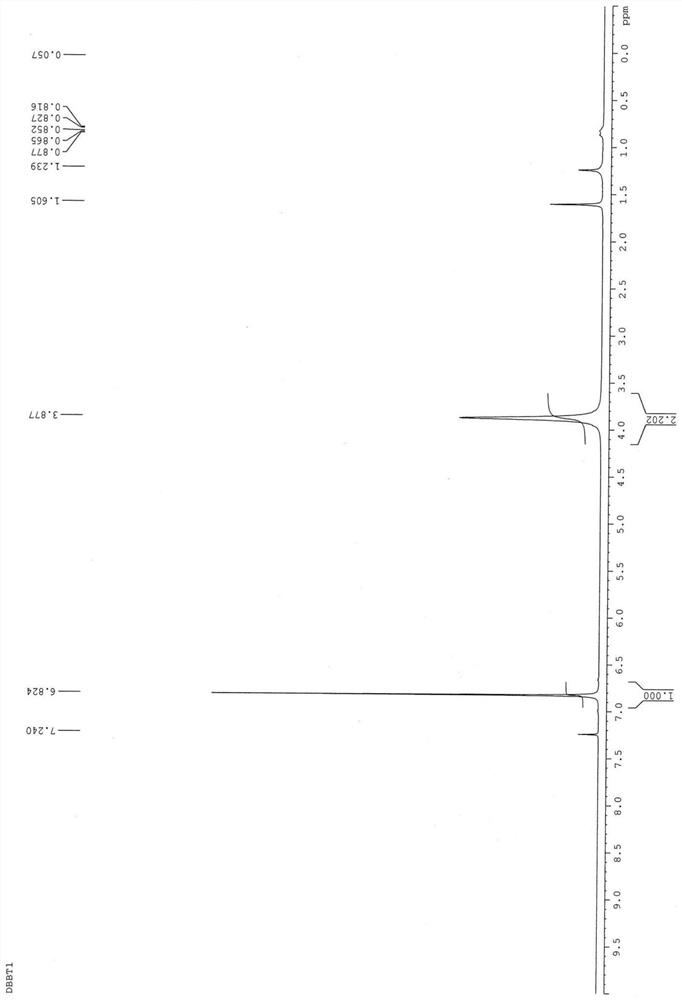
[0072] Weigh DBBT (8g, 27.2mmol) into a 500ml three-neck flask, add ethanol (120mL) and tetrahydrofuran (200mL), stir in an ice bath with a magnet until completely dissolved, and slowly add NaBH 4 (21g, 544mmol), stirred in an ice bath for 30 minutes, removed the ice bath and returned to room temperature for 1 hour, acidified the crude product, extracted three times with ethyl acetate / water, collected the organic layer and added magnesium sulfate to remove water, And the solvent was removed to obtain the product M1 as a white solid (6.5 g, 90%). The white solid M1 uses 1 HNMR (500MHz, CDCl 3 ) identification results are: δ6.82(s, 2H), 3.88(s, 4H), see figure 1 .
[0073] b. Synthesis of M2
[0074] Weigh M1 (7g, 26.3mmol) and selenium oxide (3.5g, 31.6mmol) into a 500ml three-neck flask, add ethanol (210mL) and...
Embodiment 2
[0091] Embodiment 2: the preparation of compound N3, N4
[0092]
Embodiment 3
[0093] Embodiment 3: the preparation of compound N5, N6
[0094]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com