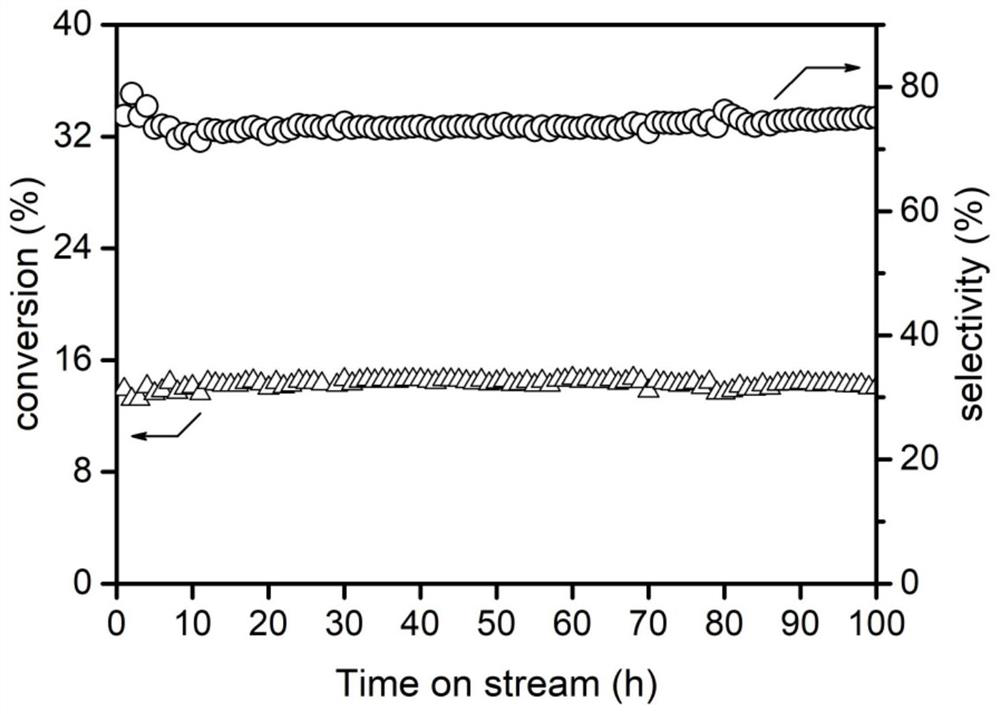
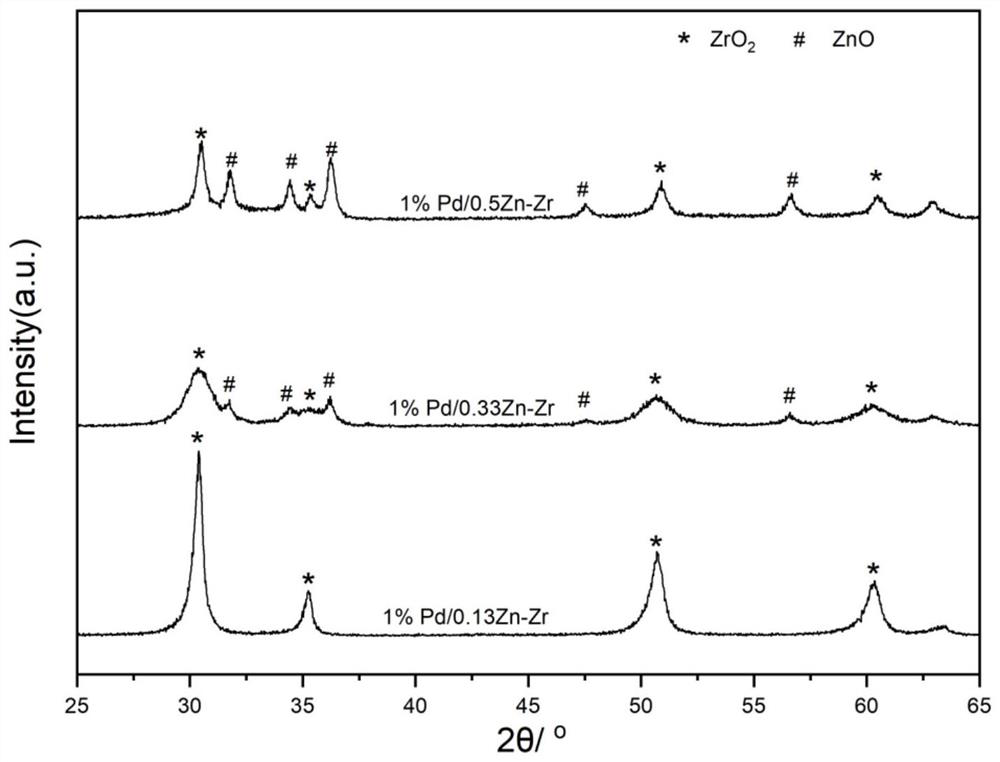
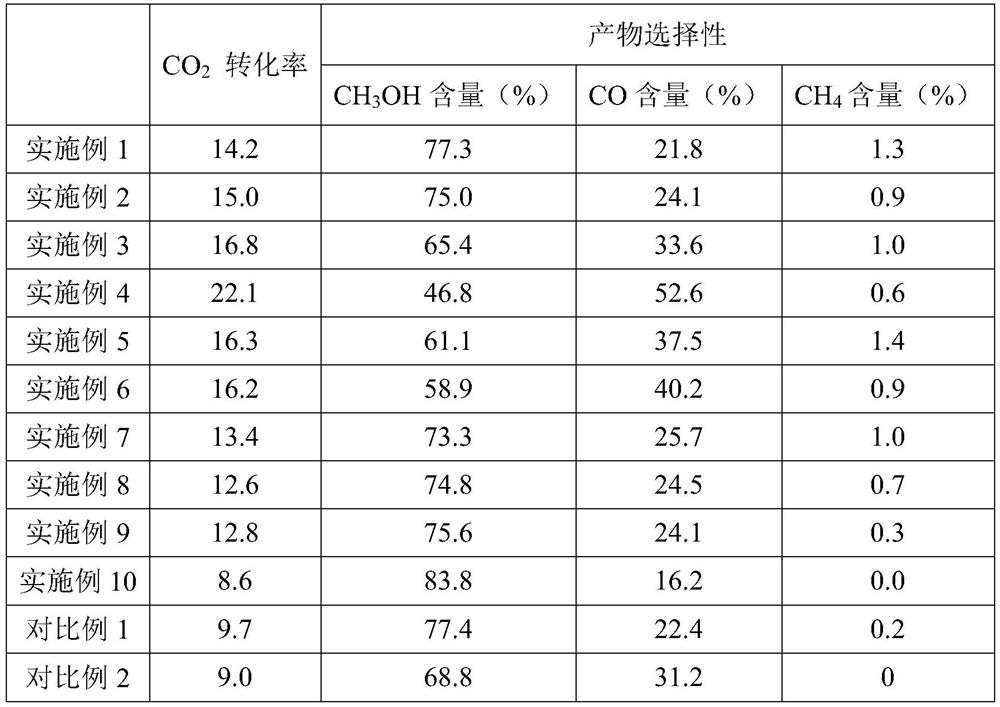
Carbon dioxide hydrogenation catalyst and preparation method and application thereof
A carbon dioxide and catalyst technology, applied in the field of catalytic conversion of carbon dioxide hydrogenation, can solve problems such as unsatisfactory conversion rate, achieve excellent catalytic performance, change catalytic performance, and good methanol yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Pd-doped ZnZr oxide solid solution (Pd-Zn x Zr 1-x O): Zinc nitrate and zirconium nitrate were dissolved in 240 mL of deionized water at a molar ratio of 0.13:0.87 to form salt solution A (the quality of the two nitrates added was 1.313 g and 12.685 g respectively), stirred at 30° C. for 1 h, Then add palladium nitrate of corresponding quality containing 0.004g Pd in the salt solution A and stir to dissolve. Then 6.660g of ammonium carbonate was dissolved in 220mL of deionized water and fully stirred and dissolved to form alkaline solution B. Transfer solution A to a round-bottomed flask and preheat it in an oil bath at 70°C, then add solution B to the round-bottomed flask at a rate of 3ml / min with a peristaltic pump under nitrogen purging, and then continue stirring at 70°C 3h. The resulting precipitate was cooled to room temperature, and the precipitate and the solution were separated under high-speed centrifugation. The precipitate was washed with deionized wate...
Embodiment 2
[0042] Pd-doped ZnZr oxide solid solution (Pd-Zn x Zr 1-x O): Zinc nitrate and zirconium nitrate were dissolved in 240 mL of deionized water at a molar ratio of 0.13:0.87 to form salt solution A (the quality of the two nitrates added was 1.313 g and 12.685 g respectively), stirred at 30° C. for 1 h, Then add palladium nitrate of corresponding quality containing 0.008g Pd in the salt solution A and stir to dissolve. Then 6.660g of ammonium carbonate was dissolved in 220mL of deionized water and fully stirred and dissolved to form alkaline solution B. Transfer solution A to a round-bottomed flask and preheat it in an oil bath at 70°C, then add solution B to the round-bottomed flask at a rate of 3ml / min with a peristaltic pump under nitrogen purging, and then continue stirring at 70°C 3h. The resulting precipitate was cooled to room temperature, and the precipitate and the solution were separated under high-speed centrifugation. The precipitate was washed with deionized wate...
Embodiment 3
[0046] Pd-doped ZnZr oxide solid solution (Pd-Zn x Zr 1-x O): Zinc nitrate and zirconium nitrate were dissolved in 240 mL of deionized water at a molar ratio of 0.13:0.87 to form salt solution A (the quality of the two nitrates added was 1.313 g and 12.685 g respectively), stirred at 30° C. for 1 h, Then add palladium nitrate of corresponding quality containing 0.020g Pd in the salt solution A and stir to dissolve. Then 6.660g of ammonium carbonate was dissolved in 220mL of deionized water and fully stirred and dissolved to form alkaline solution B. Transfer solution A to a round-bottomed flask and preheat it in an oil bath at 70°C, then add solution B to the round-bottomed flask at a rate of 3ml / min with a peristaltic pump under nitrogen purging, and then continue stirring at 70°C 3h. The resulting precipitate was cooled to room temperature, and the precipitate and the solution were separated under high-speed centrifugation. The precipitate was washed with deionized wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com