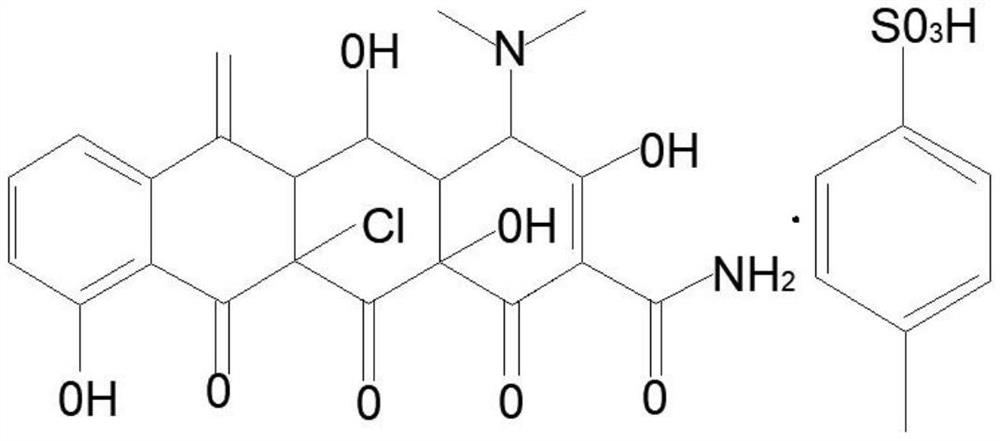
A kind of synthetic method of 11α-chloromethyloxytetracycline p-toluenesulfonate
A technology of oxytetracycline chloramphenicol and p-toluenesulfonate, which is applied in the preparation of sulfonate, chemical instruments and methods, and preparation of carboxylic acid amide, which can solve the problems of environmental pollution and high production cost, and achieve safety High, low cost, simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A kind of synthetic method of 11α-chloromethytetracycline p-toluenesulfonate, comprising the following steps:
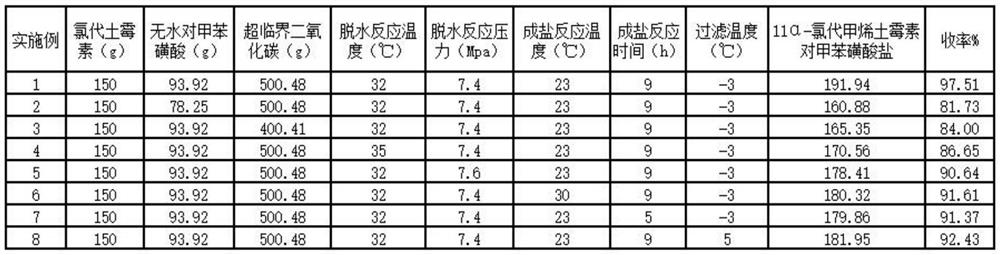
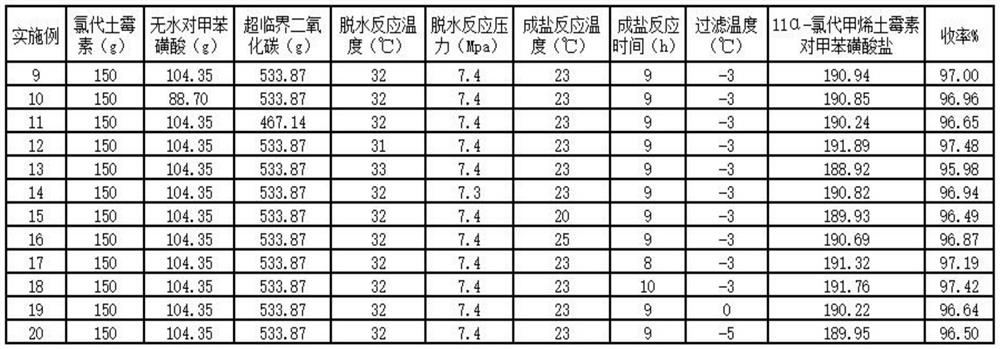
[0023] (1) Dehydration reaction: add 150g oxytetracycline and 93.92g anhydrous p-toluenesulfonic acid (that is, chloroxytetracycline and anhydrous p-toluenesulfonic acid) in a 1000ml autoclave equipped with a thermometer, stirring and pressure gauge The molar ratio of acid is 1:1.8), feed 500.48g supercritical carbon dioxide (that is, the molar ratio of chloroxytetracycline and supercritical carbon dioxide is 1:37.5), adjust the temperature to 32°C, control the pressure at 7.4MPa, and keep the temperature Stir for 5h to 8h to obtain 11α-chloromethyoxytetracycline.
[0024] (2) Salt-forming reaction: after the heat preservation is completed, the pressure is released, the temperature is lowered to 23 °C, and 600 g of anhydrous ethanol is added to the autoclave, and the temperature is kept at 23 °C and stirred for 9 hours. Drying in a vacuum oven at a low temper...
Embodiment 2
[0026] A kind of synthetic method of 11α-chloromethytetracycline p-toluenesulfonate, comprising the following steps:
[0027] (1) Dehydration reaction: add 150g oxytetracycline and 78.25g anhydrous p-toluenesulfonic acid (that is, chloroxytetracycline and anhydrous p-toluenesulfonic acid) in a 1000ml autoclave equipped with a thermometer, stirring and pressure gauge The molar ratio of acid is 1:1.5), feed 500.48g supercritical carbon dioxide (that is, the molar ratio of chloroxytetracycline and supercritical carbon dioxide is 1:37.5), adjust the temperature to 32°C, control the pressure at 7.4MPa, and keep the temperature Stir for 5h to 8h to obtain 11α-chloromethyoxytetracycline.
[0028] (2) After the heat preservation of the salt-forming reaction was completed, the pressure was released, the temperature was lowered to 23 °C, and 600 g of absolute ethanol was added to the autoclave, and the temperature was kept at 23 °C and stirred for 9 hours. Drying in a vacuum oven at a ...
Embodiment 3
[0030] A kind of synthetic method of 11α-chloromethytetracycline p-toluenesulfonate, comprising the following steps:
[0031] (1) Dehydration reaction: add 150g oxytetracycline and 93.92g anhydrous p-toluenesulfonic acid (that is, chloroxytetracycline and anhydrous p-toluenesulfonic acid) in a 1000ml autoclave equipped with a thermometer, stirring and pressure gauge The molar ratio of acid is 1:1.8), feed 500.48g supercritical carbon dioxide (that is, the molar ratio of chloroxytetracycline and supercritical carbon dioxide is 1:37.5), adjust the temperature to 32°C, control the pressure at 7.4MPa, and keep the temperature Stir for 5h to 8h to obtain 11α-chloromethyoxytetracycline.
[0032] (2) Salt-forming reaction: after the heat preservation is completed, the pressure is released, the temperature is lowered to 23 °C, and 600 g of anhydrous ethanol is added to the autoclave, and the temperature is kept at 23 °C and stirred for 9 hours. Drying in a vacuum oven at a low temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com