Andrographolide derivative for resisting influenza virus infection and preparation method thereof
A technology of andrographolide, anti-influenza virus, applied in the direction of antiviral agent, organic chemistry, etc., can solve the problems of high energy consumption, reduced synthesis yield, unfavorable amplification of production, etc., achieves broad application prospects, reduces the degree of disease, and survives. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
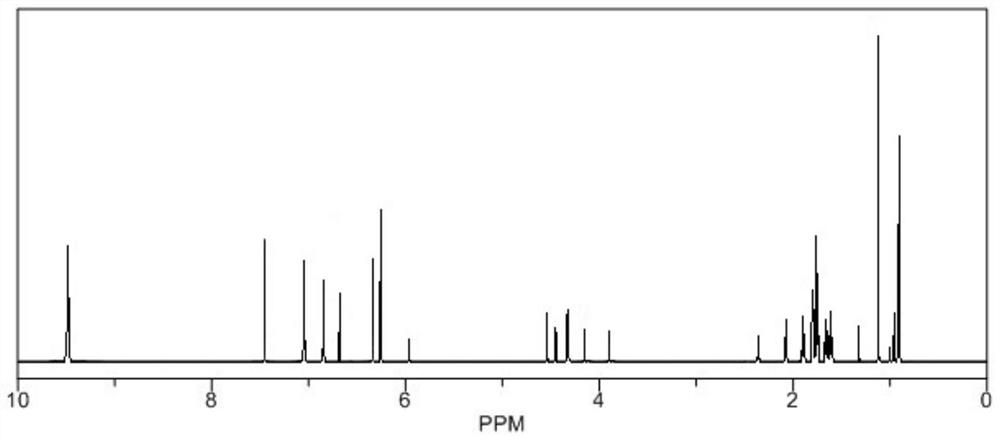
Image
Examples
Embodiment 1
[0024] (1) 20g of andrographolide was dissolved in 100mL of dichloromethane, 16.3g of p-toluenesulfonyl chloride was added, 12g of pyridine was added, reacted for 12h under ice-bath conditions, quenched with absolute ethanol, and the remaining solvent, and the residue was washed with water and saturated brine in sequence, dried over anhydrous sodium sulfate, concentrated and crystallized to obtain compound 1;
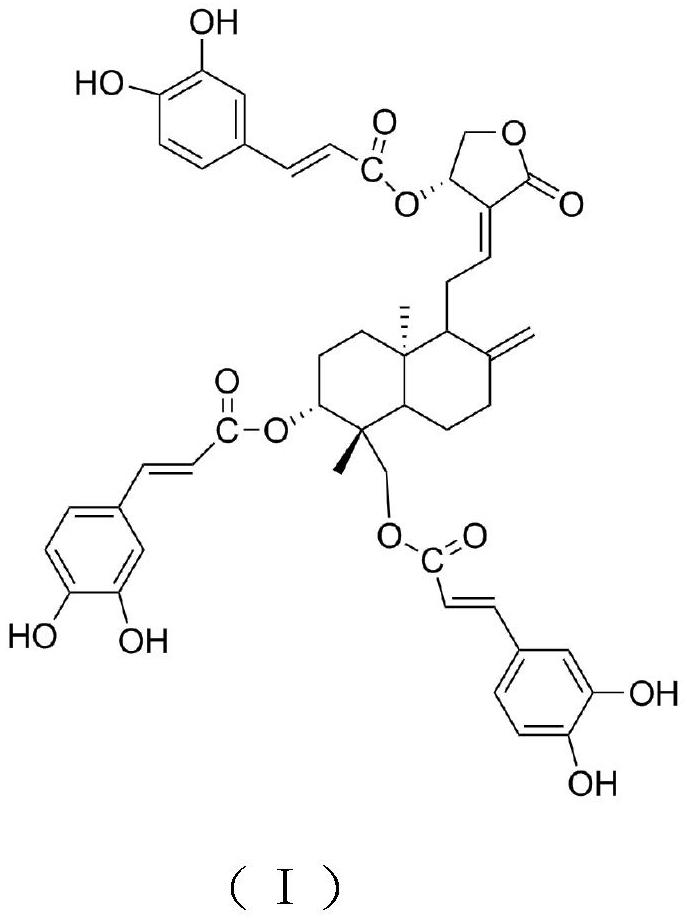
[0025] (2) Dissolve 15g of Compound 1 in 100mL of dichloromethane, add 4.6g of caffeic acid, heat to 120°C, and reflux for 4h. Sodium hydrogen solution, water washing, anhydrous calcium chloride drying, the dried crude product was dissolved in chloroform, then mixed with 200-300 mesh silica gel, the sample was separated by silica gel column chromatography (eluent: V 甲醇 :V 氯仿 =10:1), the eluate was collected, concentrated and dried to obtain andrographolide derivatives with a yield of 55.29%.
Embodiment 2
[0026] Embodiment 2 drug effect test research of compound of the present invention
[0027] (1) Effects of compounds of the present invention on lung pathological changes in mice infected with H1N1 influenza virus
[0028] Choose some of the mice of Kunming grade, group randomly, be respectively normal control group (control group 1), virus control group (control group 2), Tamiflu control group (control group 3), experimental group (embodiment 1 is the test group). drug). Mice were lightly anesthetized with ether, and infected with 15 LD50 virus drops, 0.5 mL per mouse. The mice in each group began to be administered 24 hours after the virus infection, and the administration was continued for 7 days, once a day, and each time 10mL / kg was administered. Normal control group and virus control group were given equal volume of normal saline. After the last administration, the mice were deprived of food and water for 4 hours and then dissected. The weight of the mice was weighed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com