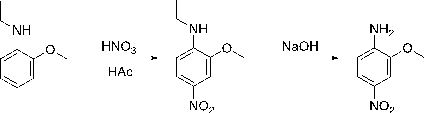
Continuous flow production process of Fast Red B
A production process, red-based technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, organic chemistry, etc., can solve the problems of poor safety, low production efficiency, and many by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
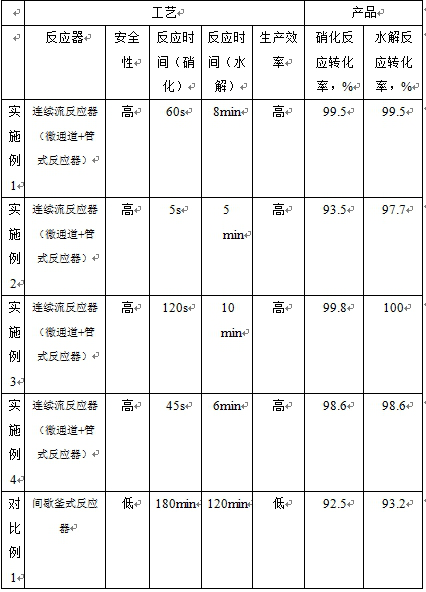
[0031] (1) Add 10mol o-methoxyacetanilide to 3850g acetic acid solution to prepare an acetic acid solution with a mass fraction of 30% o-methoxyacetanilide, 1204g concentrated nitric acid with a mass fraction of 68%, nitric acid / o-methoxy The molar ratio of acetanilide is 1.3:1. The two materials are pumped into the microchannel reactor at the same time. The total flow rate is 150g / min, the residence time is 60s, the reaction temperature is 80°C, and the pressure is 5.5bar. The nitration reaction is completed to obtain The reaction solution of oxy-4-nitro-acetanilide, detected and analyzed by liquid chromatography, the reaction conversion rate is 99.5%, and the selectivity is 85% (2-methoxy-4-nitro-acetanilide);
[0032] (2) The nitration reaction solution flows out of the microchannel reactor and enters the tubular heat exchanger, and the temperature of the reaction solution is lowered to 5°C;
[0033] (3) After cooling down, the slurry enters the pusher centrifuge to realize...
Embodiment 2
[0038] (1) Add 10mol of o-methoxyacetanilide to 6600g of acetic acid solution to form an acetic acid solution with a mass fraction of 20% o-methoxyacetanilide, 1155g of concentrated nitric acid with a mass fraction of 60%, nitric acid / o-methoxy The molar ratio of acetanilide is 1.1:1. The two materials are pumped into the microchannel reactor at the same time. The total flow rate is 40g / min, the residence time is 5s, the reaction temperature is 60°C, and the pressure is 3.5bar. The nitration reaction is completed to obtain The reaction solution of oxy-4-nitro-acetanilide was detected and analyzed by liquid chromatography, the reaction conversion rate was 93.5%, and the selectivity was 89.3% (2-methoxy-4-nitro-acetanilide);
[0039] (2) The nitration reaction solution flows out of the microchannel reactor and enters the tubular heat exchanger, and the temperature of the reaction solution is lowered to 5°C;
[0040] (3) After cooling down, the slurry enters the pusher centrifuge...
Embodiment 3
[0045] (1) Add 10mol o-methoxyacetanilide to 3064g acetic acid solution to prepare an acetic acid solution with a mass fraction of 35% o-methoxyacetanilide, 1260g concentrated nitric acid with a mass fraction of 80%, nitric acid / o-methoxy The molar ratio of acetanilide is 1.6:1. The two materials are pumped into the microchannel reactor at the same time. The total flow rate is 150g / min, the reaction temperature is 110°C, the residence time is 120s, and the pressure is 9.5bar. The reaction solution of oxy-4-nitro-acetanilide was detected and analyzed by liquid chromatography, the reaction conversion rate was 99.8%, and the selectivity was 78.0% (2-methoxy-4-nitro-acetanilide);
[0046] (2) The nitration reaction solution flows out of the microchannel reactor and enters the tubular heat exchanger, and the temperature of the reaction solution is lowered to 5°C;
[0047] (3) After cooling down, the slurry enters the pusher centrifuge to realize the continuous separation of solid a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com