Preparation method of 4-thiophenyl-o-phenylenediamine
A technology of phenylthioaniline and o-phenylenediamine, which is applied in the field of preparation of 4-phenylthioaniline-o-phenylenediamine, and can solve problems such as complicated operation, high cost, and difficult treatment of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
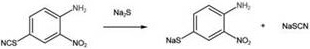
[0020] (1) Hydrolysis reaction: 2-nitro-4-thiocyanine is added 39.00 g (0.20 mol), 100 g of purified water, add 48.04 g (0.20 mol), 40-50, 48.04 g (0.20 mol), 40-50, 48.04 g (0.20 mol), 40-50 The incubation was 60 min, and the hydrolysis was 34.65 g sodium 3-nitro-4-aminephenyl thionyl sulfur, yield was 90.24%. The reaction equation is as follows:
[0021]
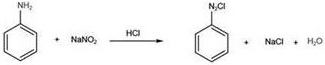
[0022] (2) Heavy nitriding reaction: 50 g of purified water was added to 250 ml of four o'cloths, and 18.61 g (0.20 mol) of aniline was added thereto, and the concentrated hydrochloric acid was 60.83 g (content of 36%, 0.60 mol), and then ammonium nitrid 15.18 was added. G (0.22 mol) was dissolved in 30 g of purified water. Added to the four flasks, 8 ° C was injected for 30 min, and the reaction was generated from 27.772 g of nitride icy, yield 98.99%. The reaction equation is as follows:
[0023]
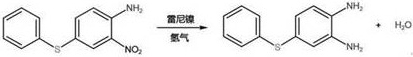
[0024] (3) Condensation Reaction: 32.64 g (0.17 mol) of 3- Nitro-4-aminephenylthiophenol sulfur sodium, 100 ml of purified w...
Embodiment 2
[0029] In this first embodiment, the second embodiment is different from the equivalent of sodium sulfide in the hydrolysis reaction, 2-nitro-4-thiocyanine 39.00 g (0.20 mol), 100g purified water, add Jiuwater in a 500 ml four-mouth bottle. Sodium sulfide was 52.84 g (0.22 mol), 40-50 ° C for 60 min, and 36.52 g of 36.52 g sodium 3-nitro-4-amine-based benzphenol was obtained, 95.11% yield.
Embodiment 3
[0031] In this first embodiment, the second embodiment is different from the equivalent of sodium sulfide in the hydrolysis reaction, 2-nitro-4-thiocyanine 39.00 g (0.20 mol), 100g purified water, add Jiuwater in a 500 ml four-mouth bottle. Sodium sulfide was 62.45 g (0.26 mol), 40-50 ° C for 60 min, and 36.87 g of 36.87 g of sodium thiosquiol sulfur sulfonol was obtained, and yield was 96.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com