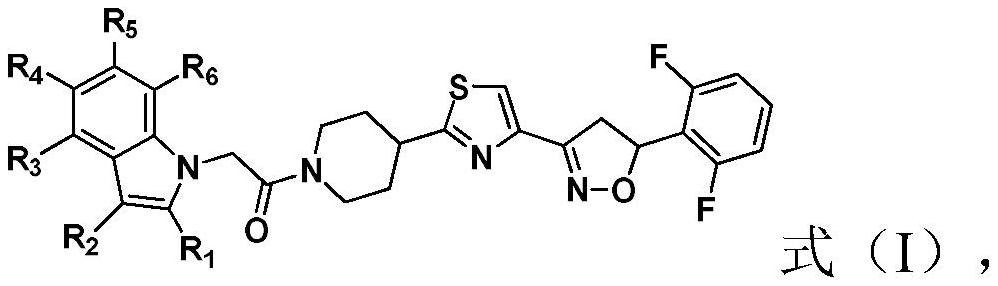
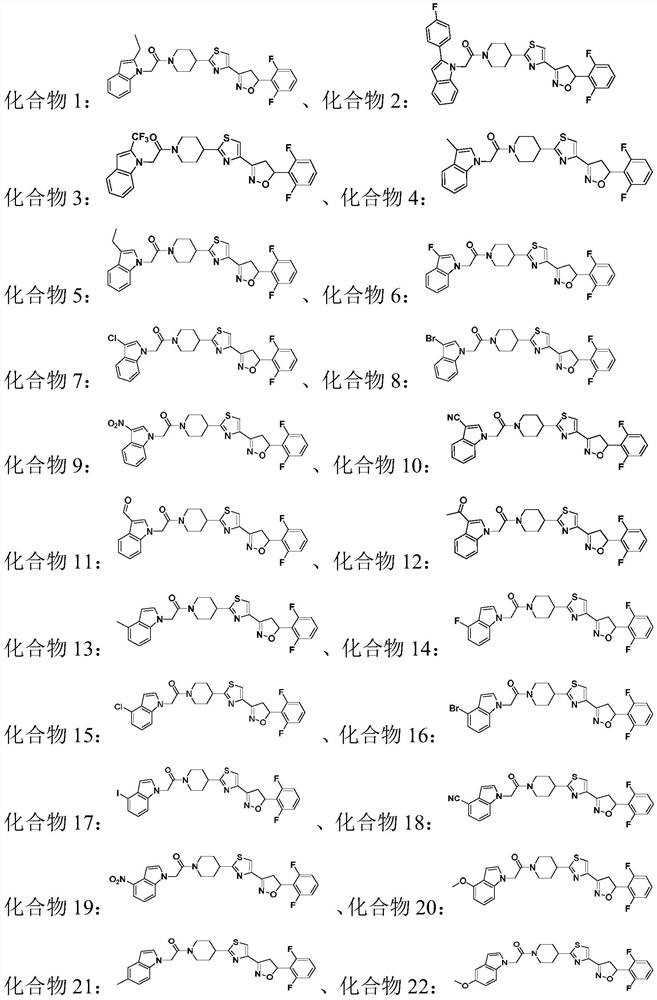
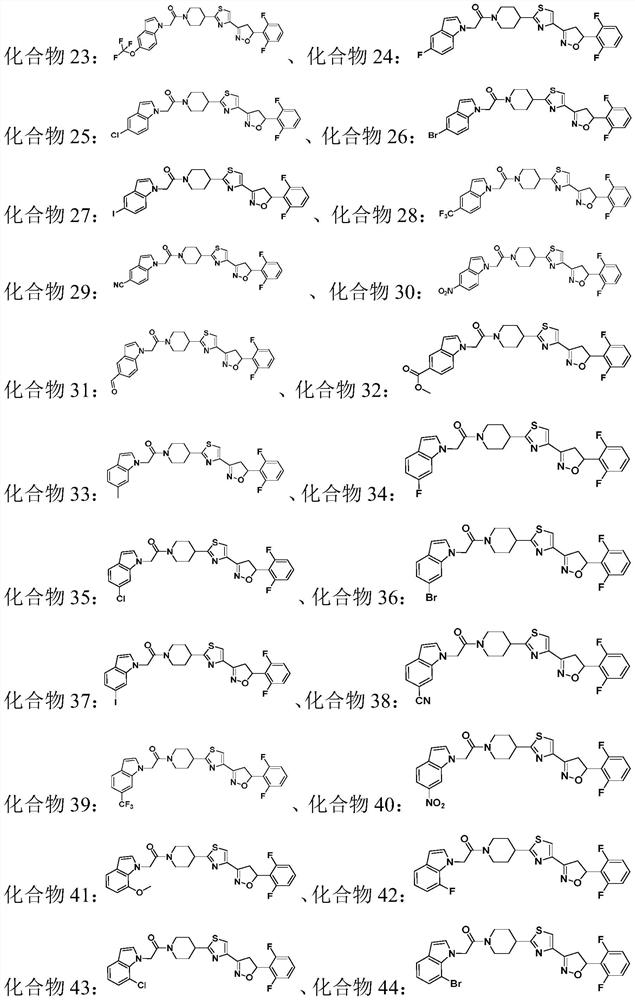
Compound containing indole ring structure, preparation method and application of compound, and bactericide
A compound, the technology of indole ring, applied in the field of pesticides, can solve problems such as irrational use, prolonged use time, pathogenic bacteria resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0069] The preparation method of the compound represented by the formula (2-6) will be described.
[0070]
[0071] Step a: At 25°C, take the compound (790.12mmol) represented by formula (2-1) and place it in a 1L two-necked flask, add 400mL of 2M diethyl ether hydrochloride solution, and slowly add tert-butyl nitrite dropwise into the reaction flask (534.62mmol, 1eq.), reacted for 3 hours. After the reaction was completed, the reaction mixture was concentrated under reduced pressure into a semi-solid, washed with 1-chlorobutane, and filtered with suction to obtain a white crystalline solid, the filtrate was concentrated, and the above process was repeated twice, and the filtered solids were combined to obtain a total of 81.10 g, to obtain the formula The compound represented by (2-2). used directly in the next reaction.
[0072] Step b: In a 500mL eggplant-shaped bottle, add magnets, the compound shown in formula (2-2) (193.48mmol, 1eq.), 250mL acetonitrile, sodium bicar...
preparation example 2
[0077] The production method of the compound represented by formula (I) will be described.
[0078]
[0079] In a 50mL eggplant-shaped bottle, add magneton and the compound (0.80mmol) represented by formula (2-7), dissolve it in 20mL ultra-dry tetrahydrofuran, cool to -5°C, add sodium hydride (1.40mmol), and react for 30 minutes, then add the compound (0.70mmol, 1eq.) shown in formula (2-6) to the system, about 1 hour, TLC monitors that the reaction of raw materials is complete, add 1.5mL water to quench, silica gel column chromatography (eluent extremely Sex is V 石油醚 :V 乙酸乙酯 =2:1~1:3) to obtain the compound shown in formula (I).
preparation example 3
[0081] The production method of some compounds represented by the formula (2-7) will be described.
[0082] The synthetic method of compound shown in intermediate formula (2-7-1):
[0083]
[0084]1-Chloromethyl-4-fluoro-1,4-diazidebicyclo[2.2.2]octanebis(tetrafluoroborate) salt (16.00mmol), lithium carbonate (32.00mmol), dichloromethane ( 26mL), water (13mL) were successively added to a round-bottom flask equipped with a stirring magnet, and indole-3-carboxylic acid (8.00mmol, 1eq.) was added, and the mixed system was stirred under ice bath for 4 hours. The completion of the reaction was monitored by TLC, moved to room temperature, diluted with water, extracted with dichloromethane, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated, and the crude product was purified by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =20:1). The compound represented by the formula (2-7-1) was obtained as a purple-black flaky solid with a yield (based on i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com