Preparation and application of acyl carboxyl sulfur nitrogen ester compound
An acyl carboxyl group and thionitroester technology is applied in the field of mineral flotation and mineral flotation collectors. performance and metal sorting selectivity, improved collection capacity, and the effect of simple operation of the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
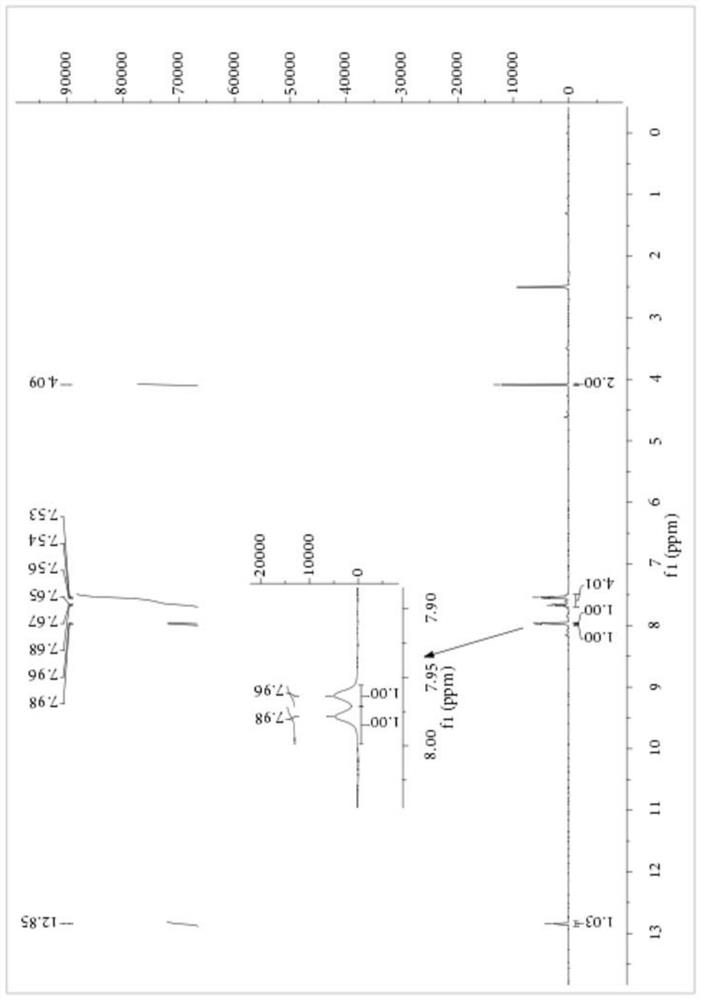
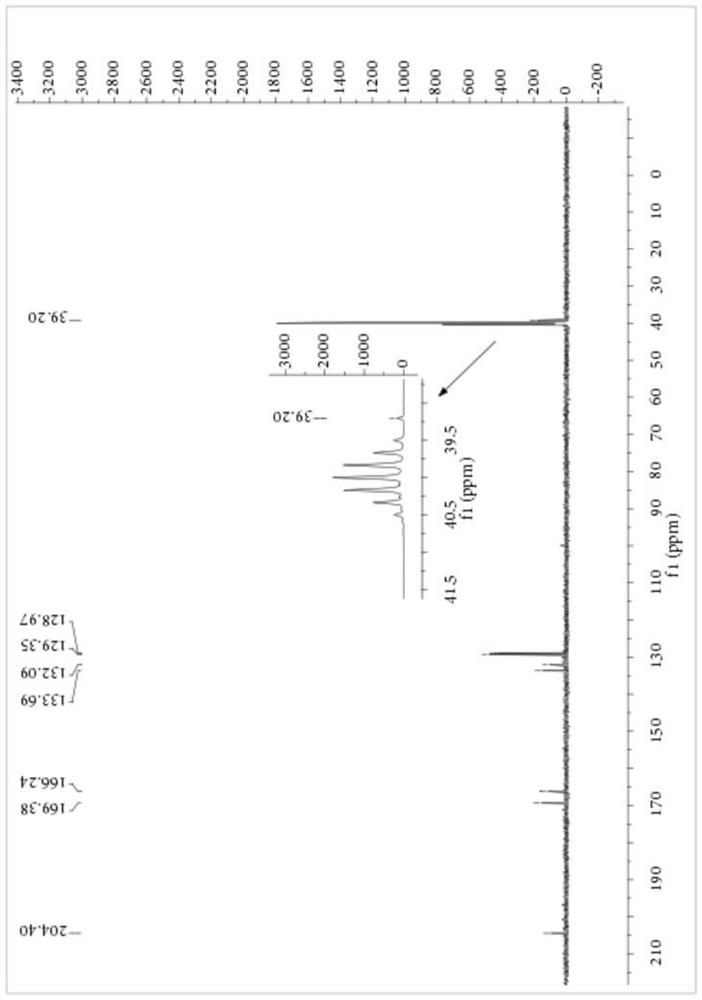
[0059] Embodiment 1: Preparation of S-carboxyethyl-N-benzoyl dithiocarbamate
[0060] Add 1.15 parts of PEG-400 with a purity of 99%, 8.27 parts of NaSCN with a purity of 98% and 80 parts of dichloromethane with a purity of 99% into a 250mL three-necked flask, stir at 10°C for 10 minutes, then 14.20 parts Benzoyl chloride with a purity of 99% was heated to 25° C. for 3.5 hours after the dropwise addition, and filtered to remove salt after the reaction, and dichloromethane was recovered by rotary evaporation. Then, 9.40 parts of 2-mercaptoacetic acid with a purity of 98% were added to the reaction vessel, and the temperature was raised to 35° C. for 4 hours to react. After completion of the reaction, petroleum ether was added to precipitate a yellow solid, which was filtered to obtain the crude product of S-carboxyethyl-N-benzoyl dithiocarbamate, the product purity was 84.56%, and the yield based on 2-mercaptoacetic acid was 88.13%. The crude product of S-carboxyethyl-N-benzo...
Embodiment 2
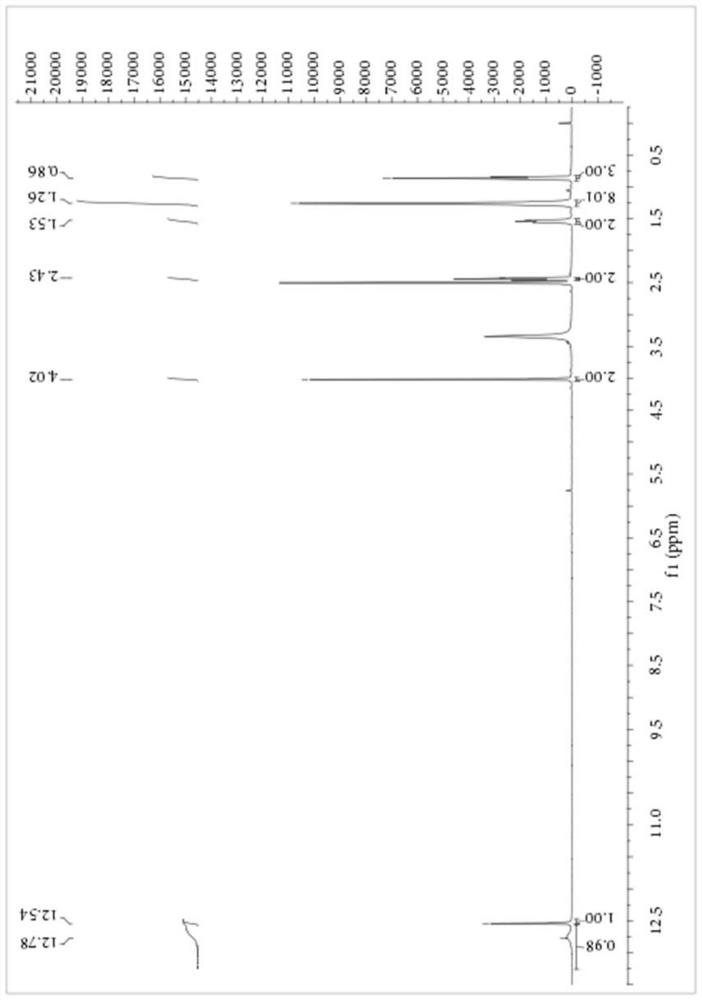
[0061] Embodiment 2: Preparation of S-carboxyethyl-N-octanoyl dithiocarbamate
[0062] Add 1.15 parts of PEG-400 with a purity of 99%, 8.27 parts of NaSCN with a purity of 98% and 80 parts of dichloromethane with a purity of 99% into a 250mL three-necked flask, stir at 10°C for 10 minutes, and then add 16.43 A portion of octanoyl chloride with a purity of 99% was heated to 40° C. for 3.5 hours. After the reaction, the salt was removed by filtration, the dichloromethane was recovered by rotary evaporation, and 9.40 parts of sodium 2-mercaptoacetate with a purity of 98% were added to the reaction vessel, and the temperature was raised to 40° C. for 4 hours. After the reaction, add sherwood oil, separate out a yellow solid, filter to get the crude product of S-carboxyethyl-N-octanoyl dithiocarbamate, the product purity is 83.45%, and the yield based on 2-mercaptoacetate sodium is 86.27%. The crude product was purified by ethanol / water recrystallization for structural analysis. ...
Embodiment 3
[0066] Example 3: Flotation of S-carboxyethyl-N-benzoyl dithiocarbamate to chalcopyrite
[0067] In S-carboxyethyl-N-benzoyl dithiocarbamate concentration of 2 × 10 -5 mol / L, the pH of the pulp is 8.0, and the concentration of foaming agent (MIBC) is 1.5×10 -4 mol / L,N 2 The flow rate is 200mL / min, and the chalcopyrite with a particle size of -0.076mm-+0.038mm is floated for 3 minutes, and the flotation recovery rate of the chalcopyrite is 96.23%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com