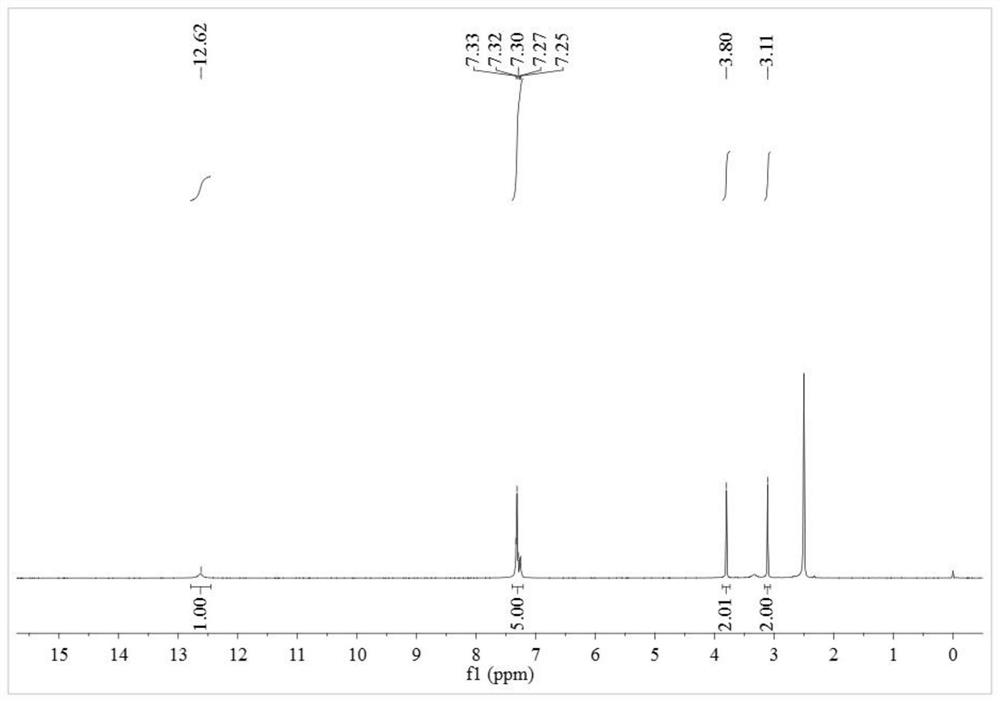
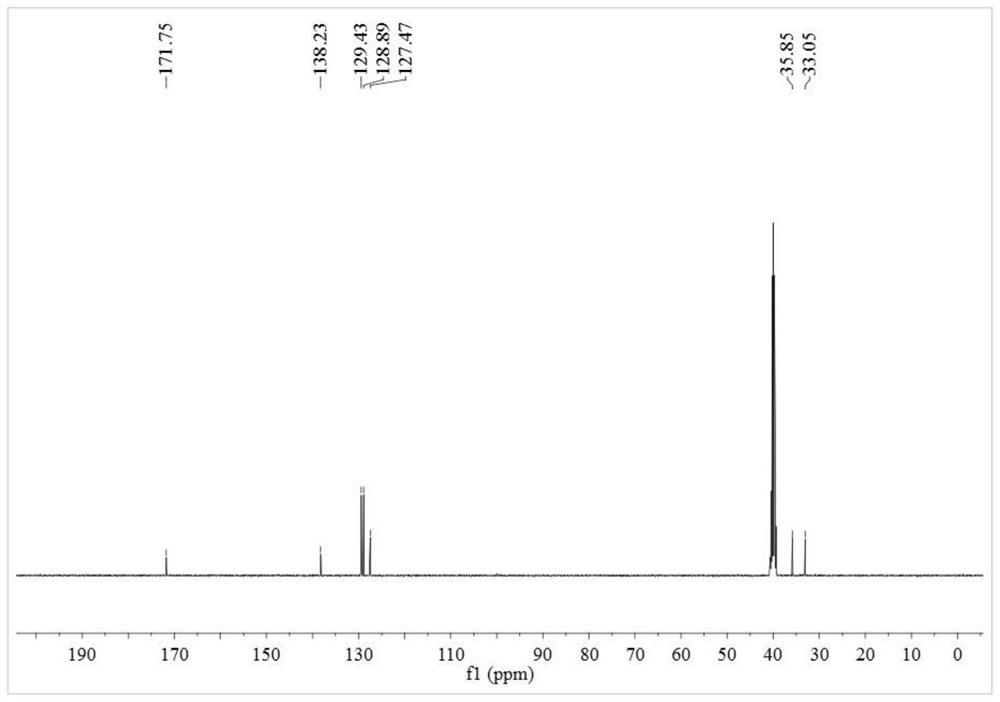
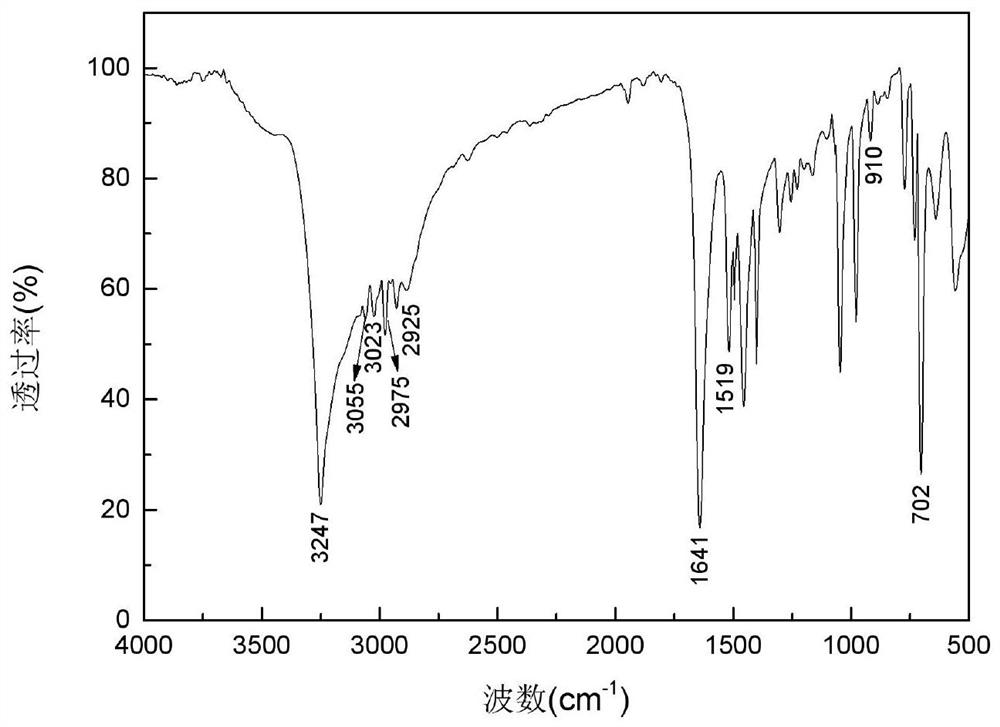
A kind of alkyl sulfide ethyl hydroxamic acid medicament and its preparation method and application
A technology of ethyl ethyl hydroxamic acid and alkyl sulfide, which is applied in the field of alkyl sulfide ethyl hydroxamic acid medicament and its preparation, and can solve the problem of weak collection ability and no alkyl sulfide ethyl hydroxamic acid. Oxymate reagent mineral flotation collector and other problems, to achieve strong chelation ability, improve hydrophobic foaming performance, and good collection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of benzyl sulfide ethyl hydroxamic acid:
[0052] Add 18.93g of 96.15% benzyl thioether acetic acid, 16.16g of 99% methanol and 2.5g of 98% concentrated sulfuric acid into a 150mL three-necked flask, raise the temperature to 75°C for 5 hours, and after cooling to room temperature, add 4.2g of 98.5 % sodium bicarbonate solid, after no bubbles are released, filter, and distill off methanol under reduced pressure to obtain methyl benzyl sulfide acetate. Add 7.76g of 99.5% hydroxylamine hydrochloride into a 150mL three-neck flask, and add 30mL of distilled water to dissolve the hydroxylamine hydrochloride. Weigh 8.33g of 96% sodium hydroxide, dissolve it with 20mL of distilled water, then add the aqueous solution of sodium hydroxide dropwise to the aqueous solution of hydroxylamine hydrochloride in an ice bath, after the dropwise addition, add benzyl sulfide to the mixture Methyl acetic acid was heated to 40°C for 4 hours. After the reaction, it was acidified w...
Embodiment 2
[0064] Preparation of benzyl sulfide ethyl hydroxamic acid:
[0065] Add 9.47g of 96.15% benzyl thioether acetic acid, 8.08g of 99% methanol and 1.3g of 98% concentrated sulfuric acid into a 100mL three-necked flask, raise the temperature to 75°C for 5 hours, and after cooling to room temperature, add 2.1g of 98 .5% sodium bicarbonate solid, after no bubbles are released, filter, and distill under reduced pressure to remove methanol to obtain methyl benzyl sulfide acetate. Add 3.88g of 99.5% hydroxylamine hydrochloride into a 100mL three-necked flask, and add 30mL of distilled water to dissolve the hydroxylamine hydrochloride. Weigh 6.59g of 85.0% potassium hydroxide, dissolve it with 20mL of distilled water, then add the aqueous solution of potassium hydroxide dropwise to the aqueous solution of hydroxylamine hydrochloride under ice-cooling, after the dropwise addition, add benzyl Methyl thioether acetate was heated to 40°C for 4.5 hours. After the reaction, it was acidified...
Embodiment 3
[0067] Preparation of dodecyl sulfide ethyl hydroxamic acid:
[0068] Weigh 18.71g of 97.30% dodecyl sulfide acetic acid, 16.16g of 99% methanol and 2.5g of 98% concentrated sulfuric acid into a 150mL three-neck flask, heat to 75°C for 4.5h, and cool to room temperature , Add 4.2g of 98.5% sodium bicarbonate solid, after no bubbles are released, filter, and distill under reduced pressure to obtain methyl dodecyl sulfide group acetate. Add 7.76g of 99.5% hydroxylamine hydrochloride and 30mL of distilled water into a 150mL three-neck flask, mix 8.33g of 96% sodium hydroxide with 20mL of distilled water, then add the aqueous solution of sodium hydroxide dropwise to the aqueous solution of hydroxylamine hydrochloride in an ice bath After the dropwise addition, add methyl dodecyl sulfide ethyl acetate to the mixture, raise the temperature to 40°C and react for 4 hours, acidify with sulfuric acid after the reaction to obtain the product of dodecyl sulfide ethyl hydroxamic acid 17.20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com