Pyrazolone-based glycoprotein-bound membrane dye as well as preparation method and application thereof
A pyrazolone-conjugated technology, which is applied in the field of cell membrane staining, can solve the problems of high concentration required, short residence time on the membrane, and large differences in specific protein expression, achieving low staining concentration and excellent staining effect , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
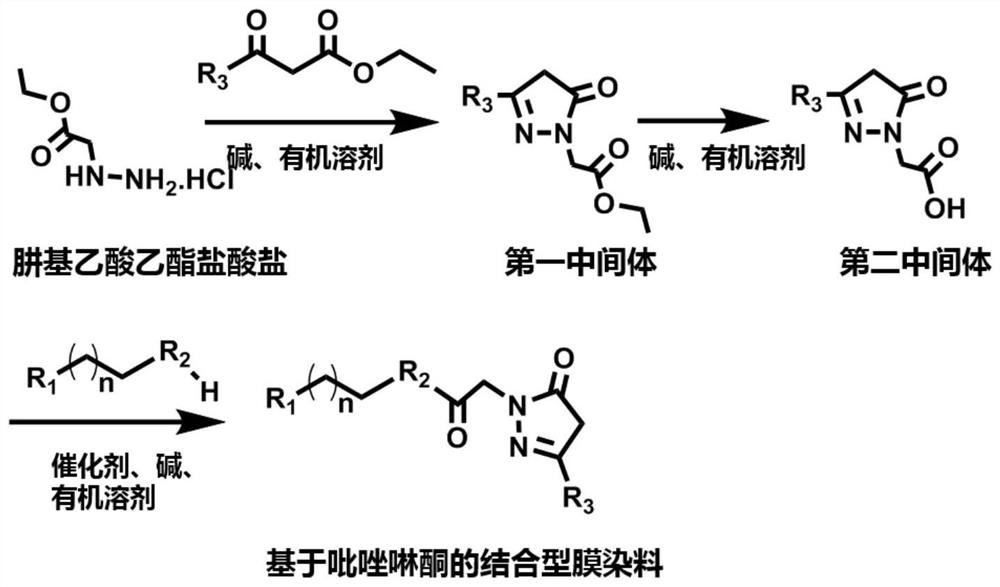
[0068] The preparation method of the fast-binding cell membrane surface glycoprotein based on the pyrazolone structure in this embodiment for long-retention cell membrane tracking imaging and as a membrane marker to distinguish co-cultured cells includes the following steps:
[0069] (1) Synthesize the dye parent molecule according to the existing method:
[0070]
[0071] (2) Synthetic intermediate 1: ethyl 2-(3-methyl-5-keto-4,5-dihydro-1H-pyrazoline) acetate
[0072] The synthetic route is as follows:
[0073]
[0074] Dissolve ethyl hydrazinoacetate hydrochloride (10mmol) in ethanol, add sodium acetate (10mmol) to it, stir for 30min, then add ethyl acetoacetate (10mmol) to it, under reflux, stir and react for 18h and then react End. After the solvent was removed by distillation under reduced pressure, ethyl acetate (100 mL) was used to redissolve and 100 mL of saturated brine was added thereto, the organic layer was separated, and extraction was continued with ethy...
Embodiment 2
[0085] Same as Example 1, only the structure of the parent dye is different, and the structure of the intermediate 2 has not changed, and its synthetic route is as follows:
[0086]
[0087] Dissolve intermediate 2 (2mmol) in 5mL DMF, add HATU (2mmol) and TMP (3mmol) to it under stirring at low temperature, add dropwise the DMF solution of dye precursor 2 (1mmol) to it half an hour later, stop after 4h of reaction reaction. After the solvent was removed by distillation under reduced pressure, the crude product was purified by silica gel chromatography, the eluent was dichloromethane:methanol=6:1 (v / v), and red solid Example 1 was obtained with a yield of 55%.
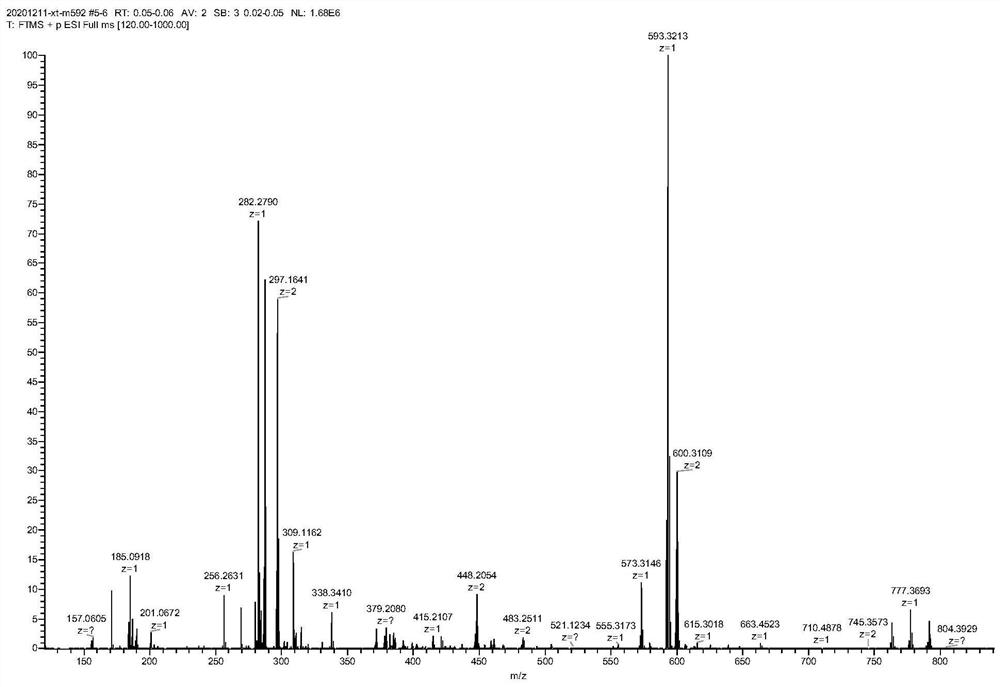
[0088] ESI-HRMS: C 31 h 40 BF 2 N 6 o 3 + [M+H] + 593.3213.
[0089] The high-resolution mass spectrum of the bound cell membrane dye prepared in Example 2 is as follows: image 3 shown.
Embodiment 3
[0091] Same as Example 1, only the structure of the parent dye is different, and the structure of the intermediate 2 has not changed, and its synthetic route is as follows:
[0092]
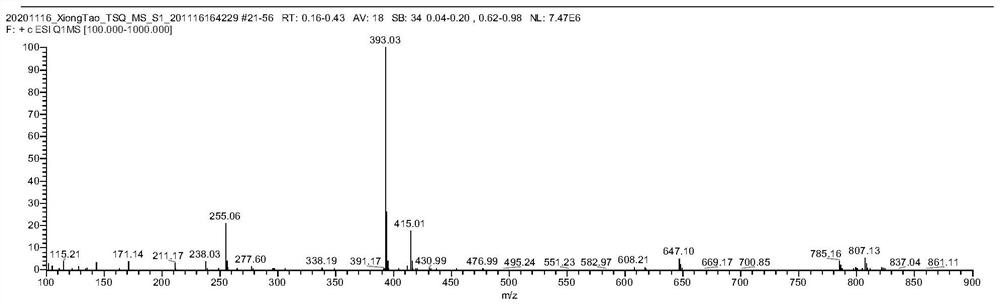
[0093] Dissolve intermediate 2 (2mmol) in 5mL DMF, add HATU (2mmol) and TMP (3mmol) to it under stirring at low temperature, add dropwise DMF solution of dye precursor 3 (1mmol) to it half an hour later, stop after 4h reaction reaction. After the solvent was removed by distillation under reduced pressure, the crude product was purified by silica gel chromatography, the eluent was dichloromethane:methanol=15:1 (v / v), and the blue solid Example 1 was obtained with a yield of 83%. ESI-HRMS: N 32 h 39 N 6 o 2 S + [M] + 571.2858.
[0094] The high-resolution mass spectrum of the bound cell membrane dye prepared in Example 3 is as follows: Figure 4 shown.
[0095] Further, in the present invention, the comparative example of dye structure design in Example 3 is used to verify the unique cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com