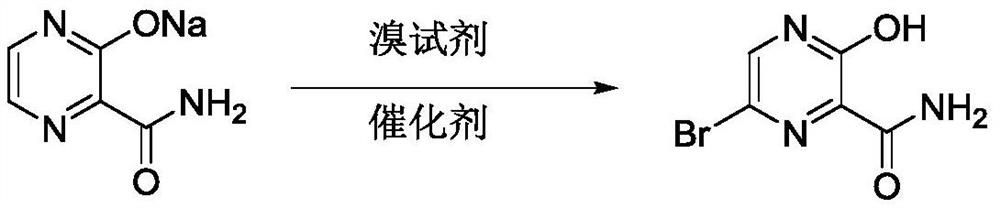
Preparation method of 6-bromo-3-hydroxy-2-pyrazinecarboxamide
A technology of pyrazine carboxamide and carbamoyl pyrazine, which is applied in the field of preparation of 6-bromo-3-hydroxy-2-pyrazine carboxamide, can solve the problems of idle instruments, reduced production efficiency, and low conversion rate of raw materials, and achieves The effect of cleaning the reaction route, simplifying the production operation and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Add methanol (160kg) and acetonitrile (600kg) to the reactor, stir and add 2-carbamoylpyrazine-3-hydroxysodium (161kg) and tetrabutylammonium bromide (16kg);
[0023] 2) Control the temperature of the system at 30-35°C, and slowly add liquid bromine to the system dropwise. After the dropwise addition, control the temperature of the system at 30-35°C, and stir for 4 hours. The bromine color of the reaction system will fade away. Use TLC to spot the reaction. whether it is completed;
[0024] 3) After the reaction is completed, cool the reaction solution to 0-10°C, and slowly add cooling water dropwise;
[0025] 4) After adding the cooling water dropwise, continue to stir and crystallize at 0-10°C for 2 hours, centrifuge, rinse with an appropriate amount of drinking water during the centrifugation process, and obtain a light yellow solid wet product;
[0026] 5) The wet product was air-dried at 70-80° C., and collected to obtain 181 kg of light yellow solid, with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com