Miconazole nitrate-containing medicinal composition containing m and preparation method thereof
A technology of miconazole nitrate and composition, which is applied in the field of pharmaceutical composition containing miconazole nitrate and its preparation, which can solve the problems of easy powder dropping of tablets and complicated production process, and achieve improved fluidity and easy access to excipients , Prescription simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A preparation method of a pharmaceutical composition containing miconazole nitrate, comprising the steps of:
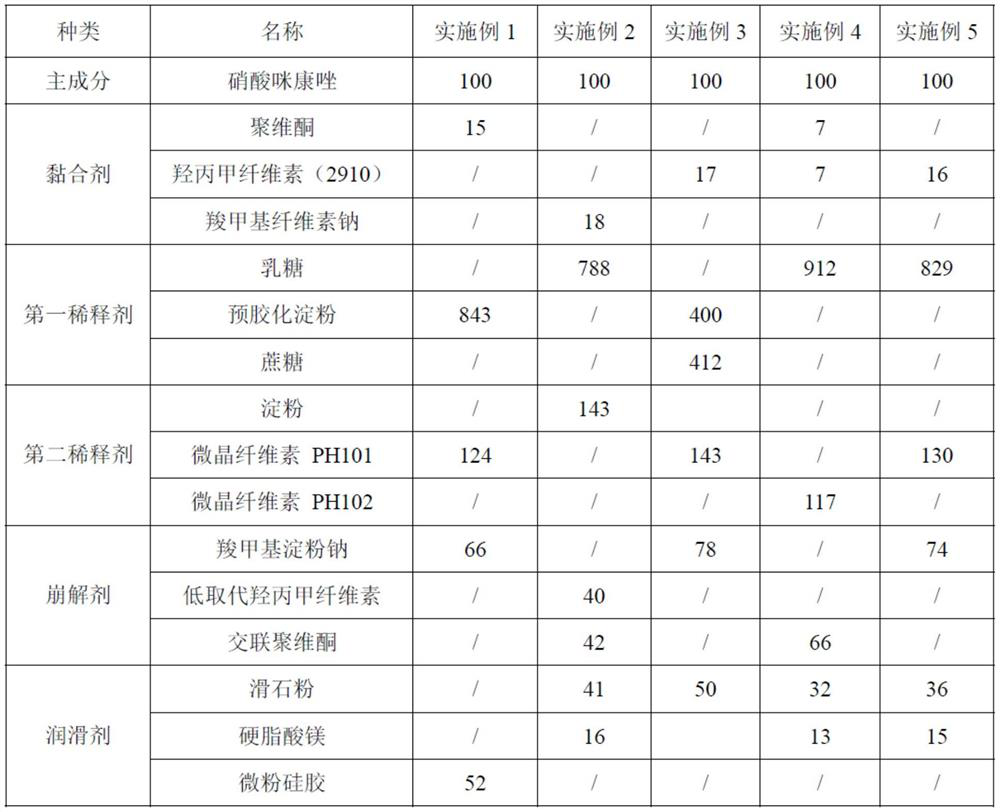
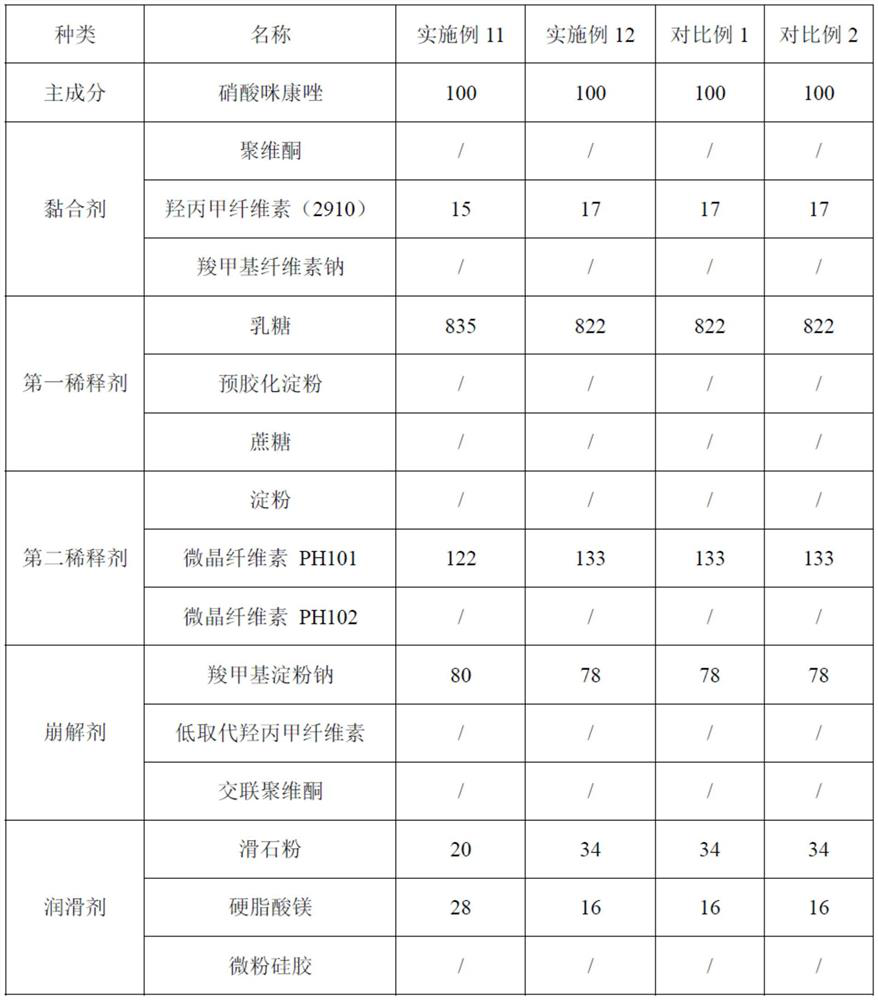
[0028] Step 1: Prepare materials according to the following weight ratio: 100 parts of miconazole nitrate, 14-18 parts of adhesive, 745-912 parts of the first diluent, 117-143 parts of the second diluent, and 66-82 parts of the disintegrant , 45-57 parts of lubricant, 96-118 parts of ethanol and 258-316 parts of purified water, set aside;
[0029] Step 2: Preparation of binder: add at least 90wt.% of the total mass of purified water and at least 65wt.% of the total mass of binder into the mixing tank, stir evenly with a mixer to obtain the binder for later use;
[0030] Step 3: mixing and sieving: mixing at least 45wt.% of the total mass of miconazole nitrate, the first diluent, the second diluent and the disintegrant, and then sieving to obtain a mixed material;
[0031] Step 4: Granulation: Put the mixed material in a rapid mixing granulator and mix for 2-5 ...
Embodiment 1
[0054] Embodiment 1: Prepared by the following method
[0055] Step 1: Prepare materials according to the following weight ratio: 100 parts of miconazole nitrate, 15 parts of povidone, 843 parts of pregelatinized starch, 124 parts of microcrystalline cellulose PH101, 66 parts of sodium carboxymethyl starch, 52 parts of micropowdered silica gel 96 parts, 96 parts of ethanol and 316 parts of purified water, standby;
[0056] Step 2: Preparation of binding agent: Add 285 parts of purified water and 10 parts of povidone into the mixing tank, and use a mixer to stir evenly to obtain the binding agent for later use;
[0057] Step 3: Mixing and sieving: Mix 100 parts of miconazole nitrate, 843 parts of pregelatinized starch, 24 parts of microcrystalline cellulose PH101124 and 30 parts of sodium carboxymethyl starch, and then use Comil to pulverize and sieve to obtain the mixed material , wherein the sieving conditions are: using a 60-mesh sieve, the rotating speed of the crushing an...
Embodiment 2
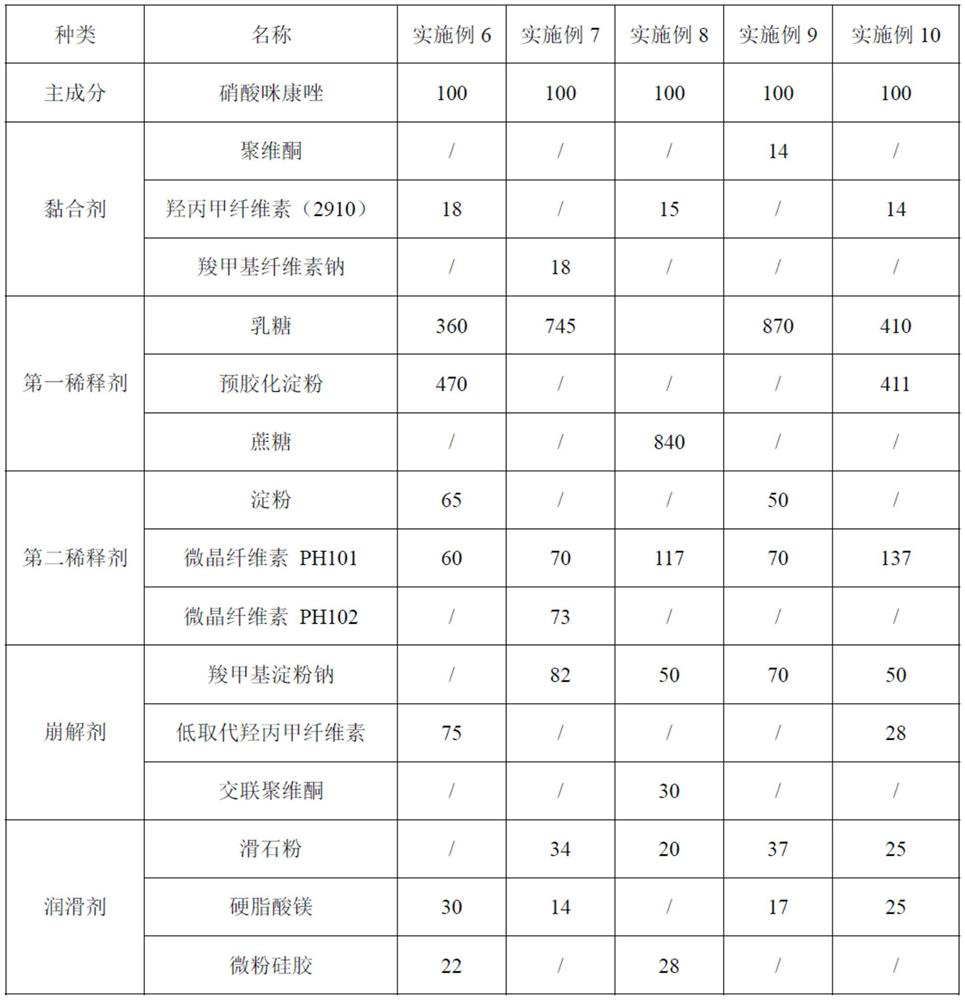
[0064] Embodiment 2: Prepared by the following method
[0065] Step 1: Prepare materials according to the following weight ratio: 100 parts of miconazole nitrate, 18 parts of sodium carboxymethylcellulose, 788 parts of lactose, 143 parts of starch, 40 parts of low-substituted hypromellose, crospovidone 42 parts, 41 parts of talcum powder, 16 parts of magnesium stearate, 100 parts of ethanol and 273 parts of purified water, set aside;
[0066] Step 2: Preparation of binding agent: Add 254 parts of purified water and 12 parts of sodium carboxymethylcellulose into the mixing tank, stir evenly with a mixer to obtain the binding agent for later use;
[0067] Step 3: Mixing and sieving: 100 parts of miconazole nitrate, 788 parts of lactose, 143 parts of starch, 20 parts of low-substituted hypromellose and 21 parts of crospovidone were mixed and passed through a comil mill The mixed material is sieved, wherein the sieving condition is: adopt a 60-mesh screen, the rotating speed of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com