Construction and application of multi-cell co-culture three-dimensional liver microsphere model
A three-dimensional model and co-cultivation technology, applied in the field of cell microspheres, can solve the problems of inability to accurately simulate the metabolic process and large differences in the two-dimensional culture environment, and achieve the effects of shortened experimental period, low cost, and simple experimental process and operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for building a three-dimensional model of multi-cell co-culture, comprising the steps of:
[0035] (1) Recovery and culture of liver cancer cells HepG2, hepatic stellate cells LX-2, and endothelial cells EA.hy926
[0036] Take out the cryopreservation tubes containing liver cancer cells HepG2, hepatic stellate cells LX-2, and endothelial cells EA.hy926 respectively, melt them in a water bath at 37°C, and centrifuge them quickly, then use a cell-specific solution containing 1% double antibody and 10% fetal bovine serum. Culture medium (HepG2 cell culture medium is MEM, LX-2 and EA.hy926 medium is DMEM); change the medium every 2 days, and when the cells cover 70-80% of the bottom of the culture dish, use trypsin The digestion solution was used for digestion. When the cell shape was observed to be oval in the microscope, and some cells were about to detach from the wall, immediately add the culture medium to stop the digestion; collect the digested cells in a 15m...
Embodiment 2
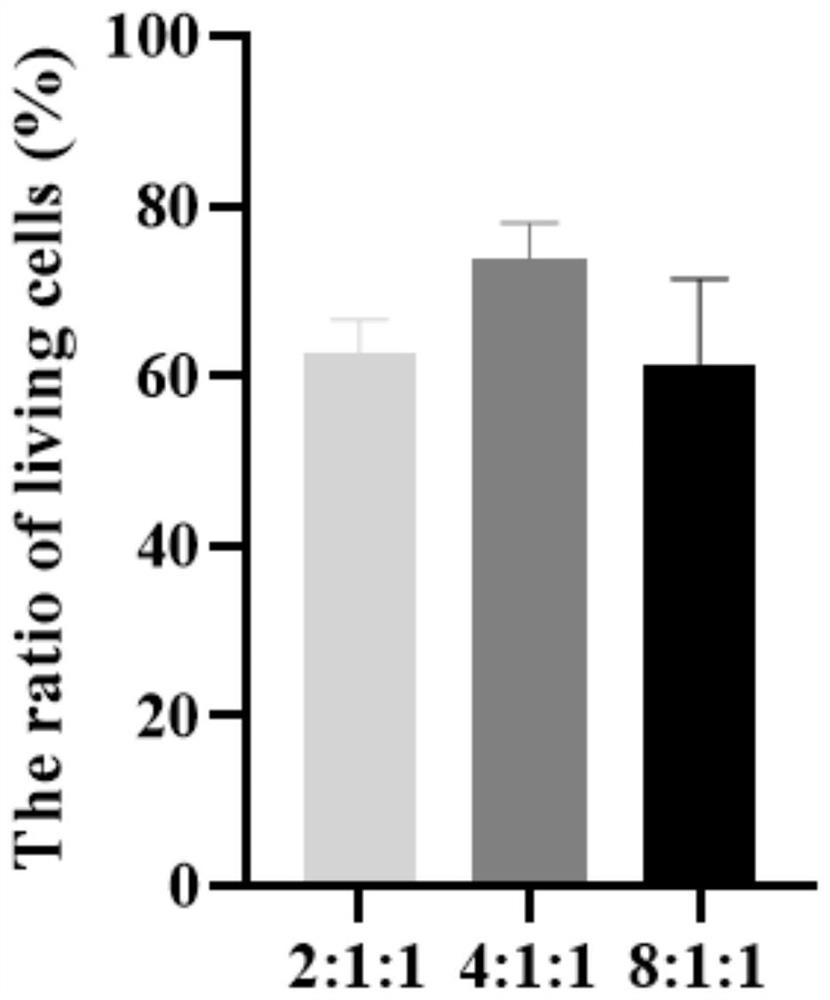
[0041] Example 2 Ratio optimization of liver cancer cells HepG2, hepatic stellate cells LX-2, and endothelial cells EA.hy926
[0042] Adjust the number ratios of liver cancer cells HepG2, endothelial cells EA.hy926, and hepatic stellate cells LX-2 in Example 1 to 2:1:1, 8:1:1; others are consistent with Example 1, and the multicellular total 3D model of culture.
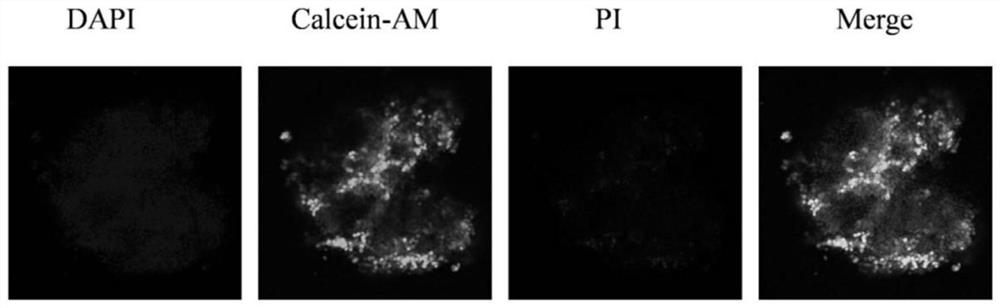
[0043] The three-dimensional model that embodiment 1 and 2 obtains is carried out cell survival situation observation, specifically:
[0044] After the shape of the microspheres is stable, aspirate the culture solution in the well plate as much as possible, add 100 μL of staining solution containing 2 μmol / LCalcein-AM and 4.5 μmol / L PI fluorescent probe to each well, and incubate at 37°C for 15 minutes; discard after the incubation is completed For the liquid in the well, add 100 μL of PBS solution to each well to wash twice, each time for 5 minutes; then add 100 μL of DAPI staining solution, incubate at 37°C for 10...
Embodiment 3
[0046] The optimization of number in the microsphere of embodiment 3
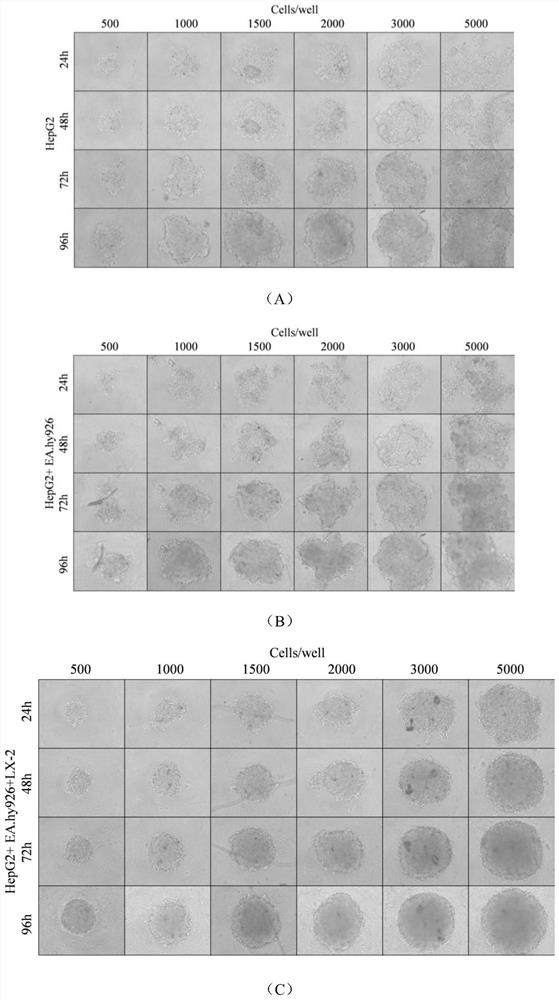
[0047]Adjust the culture time in step (3) of Example 1 so that 500 cells or 2000 cells form a sphere, and the others are consistent with Example 1 to obtain a three-dimensional cell model.
[0048] The obtained three-dimensional cell model was observed at 3, 9, 15, and 21 days and the area of the sphere was quantified. The results are as follows: Figure 4 , it can be seen that: 500 cells formed spheres due to large differences and fluctuations between samples caused by human manipulation, and the data was not stable; 2000 cells obtained microspheres after 3 days, and the area of some spheres exceeded 20 000 μm 2 , the excessive volume of the sphere may cause the cells in the middle of the microsphere to lack nutrients and oxygen, and eventually die; therefore, select 1000 cells to form a sphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com