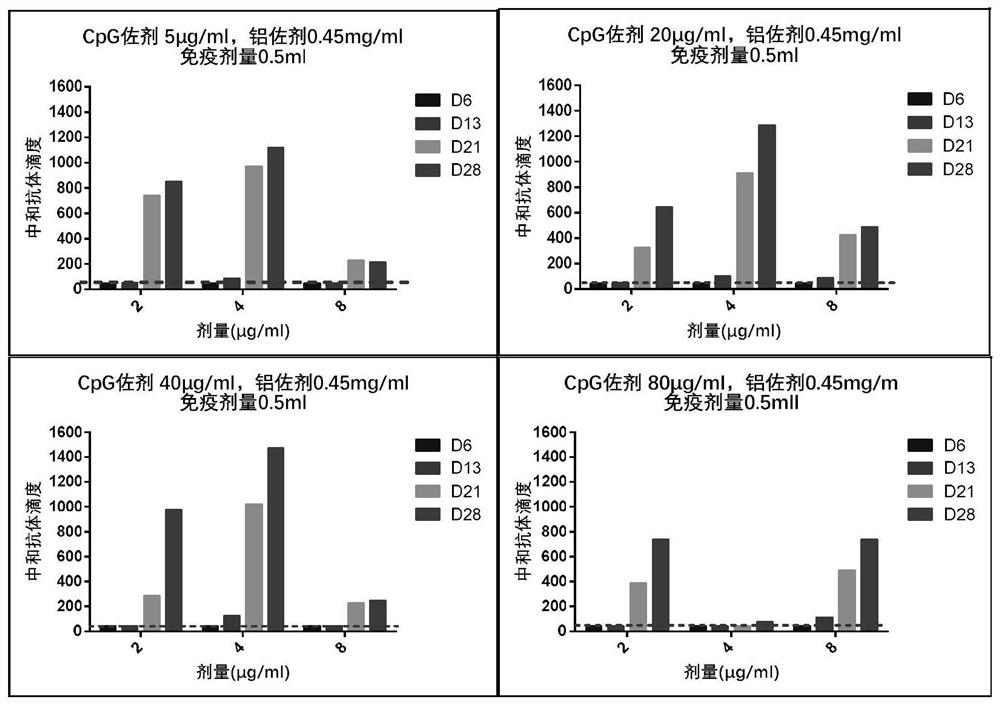
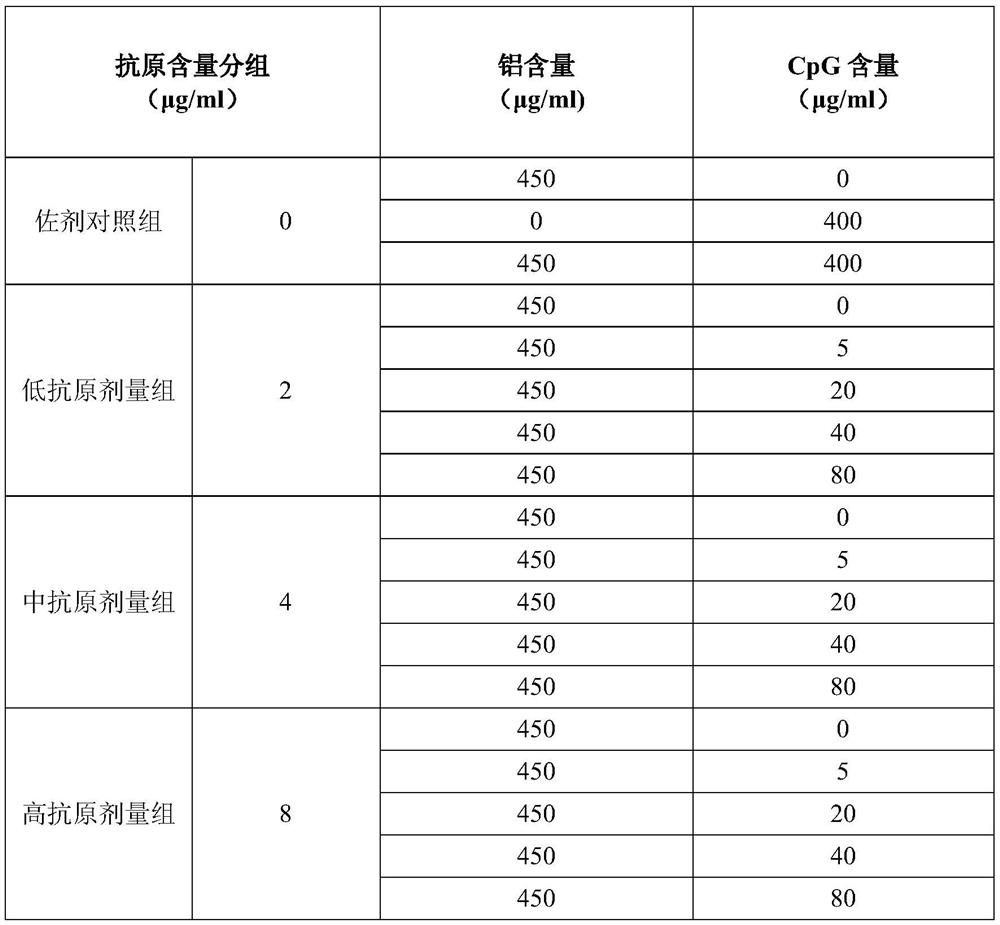
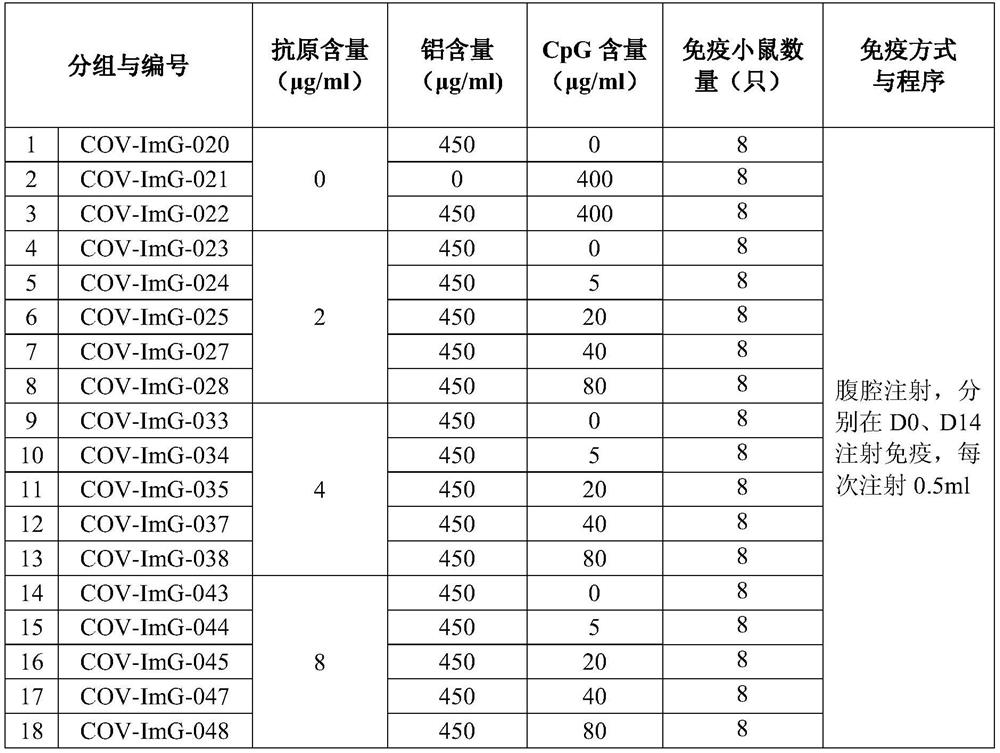
Preparation method of novel dual-adjuvant coronavirus inactivated vaccine and application thereof
A coronavirus and inactivated vaccine technology, applied in biochemical equipment and methods, viruses, antiviral agents, etc., can solve the problems of insufficient immune effect and immune time, low neutralizing antibody value, etc., to improve the ability of autoimmune response , clear ingredients, and the effect of improving the stability of the vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The present invention relates to a kind of preparation method of novel adjuvant novel coronavirus inactivated vaccine, described preparation method comprises the following steps:
[0017] ① Preparation of cell matrix and virus fluid for production;
[0018] ② Virus inactivation and verification;
[0019] ③ Preparation of inactivated vaccines;
[0020] ④ Preparation of semi-finished products and finished products.
Embodiment 1
[0021] Embodiment 1: Preparation of cell matrix and virus liquid for production:
[0022] ① Cell resuscitation: Take out the corresponding number of Vero cells (purchased from ATCC, number CCL-81) from the liquid nitrogen tank, and after checking, resuscitate them in a T25 cell culture bottle (purchased from Corning), and the medium contains 20% neonatal The 199 medium of bovine serum (the 199 medium was purchased from Beijing Tianxinhe Biotechnology, and the newborn bovine serum was purchased from Hangzhou Tianhang Biology) was placed in a constant temperature incubator at 37°C for cultivation until the cells filled a dense monolayer.
[0023] ②Cell passage: Passage the Vero cells covered with a dense monolayer, wash the cells slowly with an appropriate amount of 0.01M PBS solution (homemade), then add an appropriate amount of 0.25% trypsin digestion solution (purchased from Gbico) for digestion, and mix well into a cell suspension. Divide the cell suspension into correspond...
Embodiment 2
[0026] Embodiment 2: Harvesting and inactivation of virus liquid
[0027] ① Observe the lesions of the cell factory. After the lesions of the cell factory reach 50%-80%, put the cell factory into the refrigerator at 2-8°C to pre-cool to 4-8°C, and then add the diluted β- Propanolactone (purchased from SERVA) was placed at 2-8°C for further inactivation for 20 hours.
[0028] ② After the inactivation is completed, according to the total amount of harvested liquid and the liquid volume in the collection bucket, the inactivation sampling is carried out at a liquid volume of not less than 0.1% in each batch. The sample volume is sampled according to different harvesting containers, inoculated into Vero cells, and blind Passage 3 generations, pass 1 generation every 4 to 5 days, and check the virus by cytopathic method for each generation, and the results should all be negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com