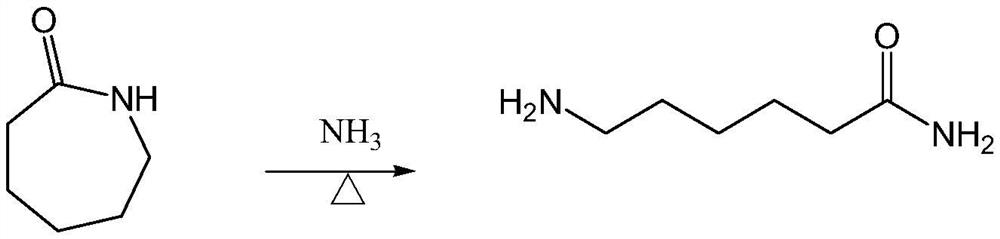
Two-step preparation method of 6-aminocapronitrile
A technology of aminocapronitrile and aminocapronamide is applied in the field of preparing hexamethylenediamine key intermediate 6-aminocapronitrile by two-step method, and can solve the problems of affecting the operation stability of the device, increasing the polymer, and coking of the catalyst.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
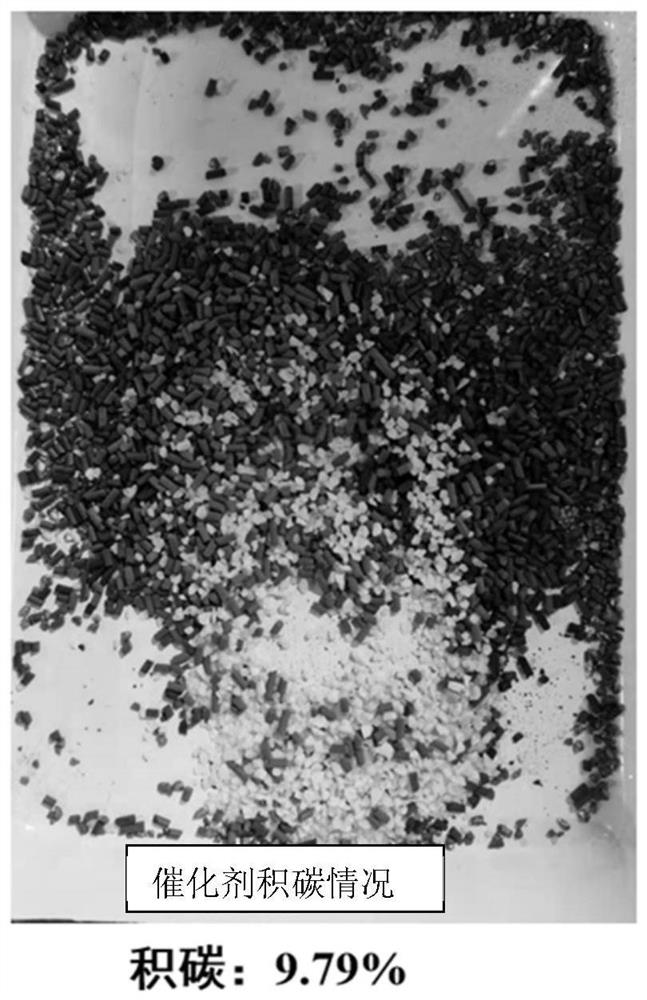
[0059] Weigh 22.63g (0.2mol) of caprolactam into a 500ml autoclave, then add 34g of toluene, start stirring and heat up to 60±2°C to dissolve. After the ammonia gas passes through the preheater, the temperature reaches 150°C, and it is passed into the pressure kettle. The temperature of the material in the reactor continues to rise to 200-250°C, and the hot ammonia gas is continuously fed in, with a back pressure of 2.5MPa. After stirring for about 20 hours, the temperature is reduced and the pressure is released. Obtain 59g of toluene solution of the crude product of 6-aminocaproamide, and the reaction process consumes 20g of ammonia gas in total. After testing, the mass percentage of 6-aminocaproamide is 72.18%, the conversion rate of caprolactam is 73.1%, and the selectivity of 6-aminocaproamide is 95.3%. The detection results of the polymerization by-products are shown in Table 1 below.
[0060] Transfer the toluene solution of the crude 6-aminocaproamide to a pressure-re...
Embodiment 2
[0062] Weigh 22.63g (0.2mol) of caprolactam into a 500ml autoclave, then add 68g of acetonitrile, start stirring and heat up to 30±2°C to dissolve. After the ammonia gas passes through the preheater, the temperature reaches 250°C, and it is passed into the pressure kettle. The temperature of the material in the reactor continues to rise to 300-350°C, and the hot ammonia gas is continuously fed in, with a back pressure of 3.0MPa. After stirring for about 20 hours, the temperature is released and the pressure is released. Obtain 89g of acetonitrile solution of the crude product of 6-aminocaproamide, and consume 31g of ammonia gas in the reaction process. After testing, the mass percentage of 6-aminocaproamide is 83.94%, the conversion rate of caprolactam is 82.7%, and the selectivity of 6-aminocaproamide is 97.1%. The detection results of the polymerization by-products are shown in Table 1 below.
[0063] Transfer the acetonitrile solution of crude 6-aminocaproamide to a pressu...
Embodiment 3
[0065] Weigh 22.63g (0.2mol) of caprolactam into a 500ml autoclave, then add 25g of chlorobenzene, start stirring and heat up to 70±2°C to dissolve. After the ammonia gas passes through the preheater, the temperature reaches 200°C, and it is passed into the pressure vessel. The temperature of the material in the reaction vessel continues to rise to 300-350°C, and the hot ammonia gas is continuously introduced, with a back pressure of 3.0MPa. After stirring for about 13 hours, the temperature is reduced and the pressure is released. Obtain 46g of crude chlorobenzene solution of 6-aminocaproamide, and consume 23g of ammonia gas in the reaction process. After testing, the mass percentage of 6-aminocaproamide is 75.51%, the conversion rate of caprolactam is 76.5%, and the selectivity of 6-aminocaproamide is 95.7%. The detection results of the polymerization by-products are shown in Table 1 below.
[0066] Transfer the acetonitrile solution of the crude product of 6-aminocaproamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com