Allopurinol sustained-release capsule
A technology of sustained-release capsules and allopurinol, applied in the field of pharmacy, can solve problems such as poor stability, high viscosity of shellac, and poor roundness of pellets, and achieve small batch-to-batch differences, complete quality standards, and high-quality products. good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
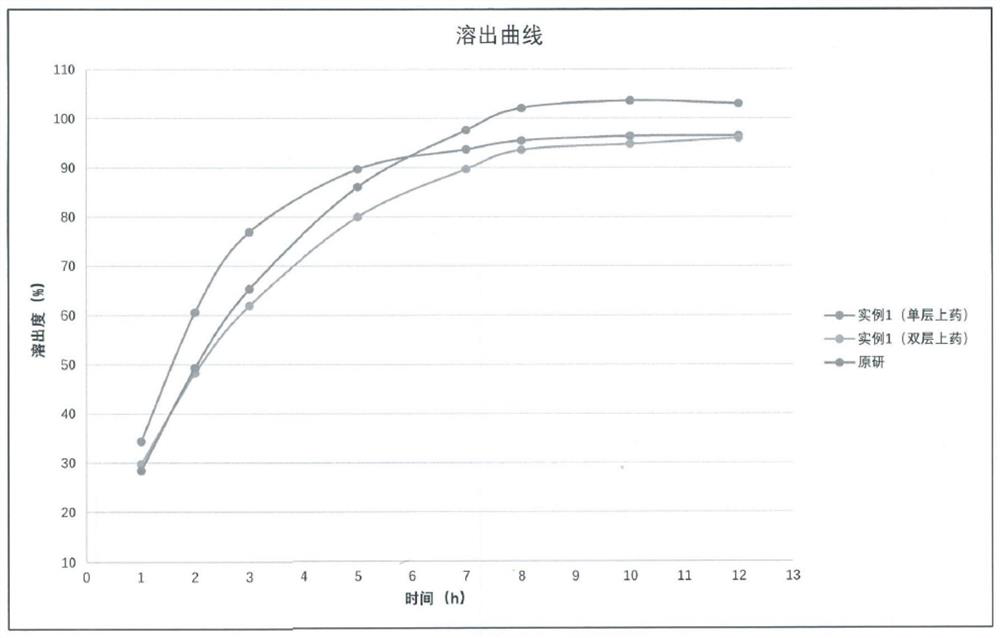
[0064] Embodiment 1, monolayer drug application
[0065] Sustained release material: povidone K90+stearic acid
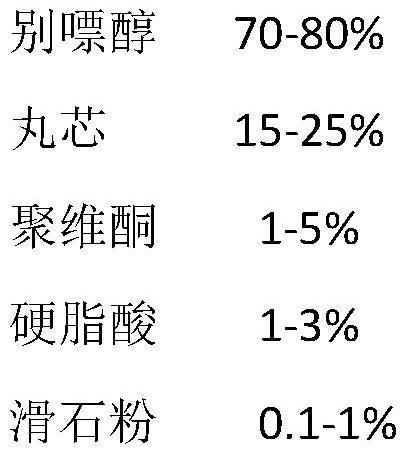
[0066]
[0067] Process:
[0068] 1. Material weighing: Weigh allopurinol, sucrose core, povidone K90, and stearic acid in prescribed dosage.
[0069] 2. Suspension preparation: Dissolve povidone K90 in 80% ethanol-water and stir well. Add stearic acid and allopurinol, stir evenly, then homogenize for 3 minutes, prepare solid content: 25%,
[0070] 3. Coating and applying medicine
[0071] Pour the ball core into the centrifugal coating machine, adjust the parameters, set the blast frequency: 30-45%, turn on the air inlet heating, set the inlet air temperature at 55-75°C, the host speed at 330-400rpm, and the atomization pressure at 0.06- 0.15Mpa, peristaltic pump speed: 20-35rpm, gradually spray the suspension liquid onto the rotating ball core in the pot, control the spray speed, air volume and air temperature, and keep the material dry and wet properly. A...
Embodiment 2
[0072] Embodiment 2, double-layer drug application
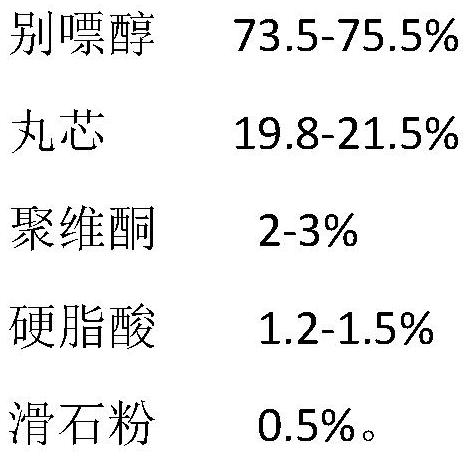
[0073]
[0074] Process:
[0075] 1. Material weighing: Weigh allopurinol, sucrose core, povidone K30, and stearic acid in prescribed dosage.
[0076] 2. Suspension solution preparation:
[0077] ①Inner liquid medicine: add povidone K30, raw material medicine, stearic acid (if any) into 80% medicinal ethanol solution, 80% ethanol, solid content: 25%, fully stir evenly, use a mixing homogenizer After homogenizing for 2-3 minutes, stir slightly mechanically for use.
[0078] ② Outer liquid medicine: Dissolve povidone K30, raw material medicine and stearic acid in 80% medicinal ethanol solution, solid content: 25%, stir well and homogenize with a mixing homogenizer for 2-3 minutes, Stir lightly mechanically and set aside.
[0079] 3. Coating and applying medicine
[0080] ① Turn on the operating equipment, set the inlet air temperature to 50-75°C, the blast air volume: 20-45%, and the host speed to 330rpm.
[0081] ②Do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com